The Aptima Combo 2® assay
Be confident in your testing results with the Aptima Combo 2® assay for CT/NG
An updated version of the Aptima Combo 2® assay, able to detect the recently emerged variants of Chlamydia trachomatis (CT), is now CE-marked and available for use in Europe.
Variants are a natural biological occurrence, and microorganisms that cause sexually transmitted infections (STIs) have been known to evolve over time. At Hologic, we are committed to updating our products as new pathogens are identified and existing ones evolve. As such, on learning of the existence of new CT variants we acted immediately to update the Aptima Combo 2 assay.
Working closely with scientific experts, laboratories, regulators and public health agencies we were able to develop a solution which not only detects the recently emerged variants of CT, but due to dual CT detection probes may also provide additional diagnostic protection against future genetic variants within the Aptima Combo 2 probe region.1
Want to find out more about the updated Aptima Combo 2 assay?
Excellent detection across specimen types
The Aptima Combo 2 assay provides an upfront biological advantage compared with assays that detect DNA as there are up to 1,000-fold more rRNA copies per cell compared to DNA.2
The assay delivers excellent performance and high sensitivity by targeting rRNA across a broad range of samples types:
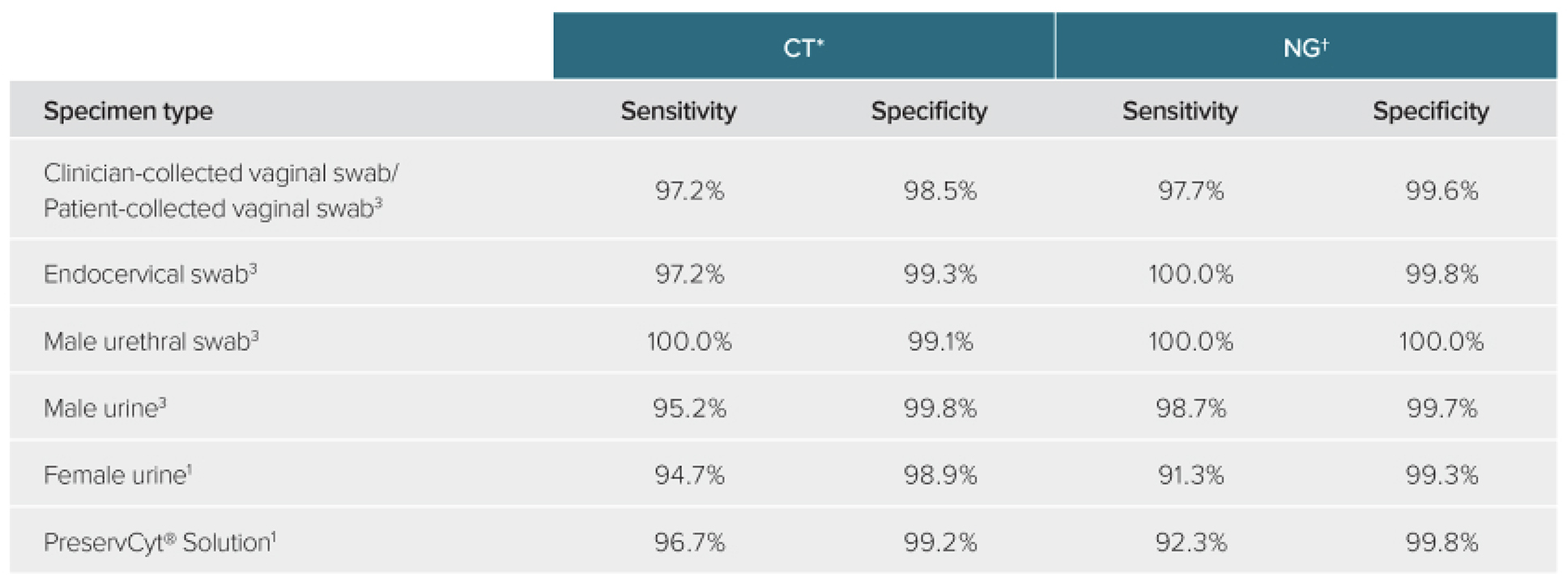
*The infected status algorithm was based on assay results from AC2 Tigris®, ACT Tigris and AC2 Panther®.
† The infected status algorithm was based on assay results from AC2 Tigris, ANG Tigris and AC2 Panther.
The Aptima Combo 2 assay demonstrates up to 100% sensitivity and specificity in detecting CT/NG from throat and rectal swabs.1
The Aptima® STI and Vaginitis assay menu
We know that accurate STI and Vaginitis testing and treatment is critical to preserving the well-being of patients. As such, we are proud to have the world’s most comprehensive portfolio of fully-automated STI and Vaginitis assays run on a high-throughput molecular platform.
One Sample. Multiple results.
Aptima assays can support any lab's growth, by offering detection of up to SEVEN infections and disease states with just one vaginal swab sample: BV, Candida species, Candida. glabrata, TV, chlamydia, gonorrhea and Mycoplasma genitalium. The Aptima® STI and Vaginitis assay menu includes:
Our diverse STI and Vaginitis testing menu allows lab personnel to deliver results to healthcare providers with confidence, empowering informed choices when it comes to patient care. |
 |
With Hologic, you can be sure you have All Bases Covered for your STI and Vaginitis testing
Contact our team today to find out more about the Aptima STI and Vaginitis assay menu, including the updated Aptima Combo 2® assay. Please provide your details below and a member of our team will be in touch.


