Respiratory Viruses Are Always Circulating: The Season That Lasts All Year

Understanding the changing viral prevalence throughout the year can help laboratories offer the appropriate tests for detecting and differentiating viruses.
The fall and winter seasons are often the times of year most people associate with experiencing respiratory illness, especially influenza A/B (flu), one of the most prevalent respiratory infections worldwide.1,2 However, these months are simply just the peak time of year for certain, more familiar, viruses. A wide range of viruses that cause respiratory symptoms are occurring year-round.2
One reason respiratory diseases are a perennial threat is that there are several different viruses responsible for causing them, including:3
- Influenza (flu) A/B
- Respiratory syncytial virus (RSV) (A and B)
- Severe acute respiratory syndrome (SARS-CoV-2), which causes COVID-19
- Human parainfluenza viruses (HPIVs) 1-4
- Adenoviruses (AdV)
- Human metapneumovirus (hMPV)
- Rhinovirus (RV)
As seen in the figure below, the prevalences of these viruses increase and wane throughout the year and, perhaps more importantly, there can be several viruses circulating simultaneously.4
The respiratory virus calendar4
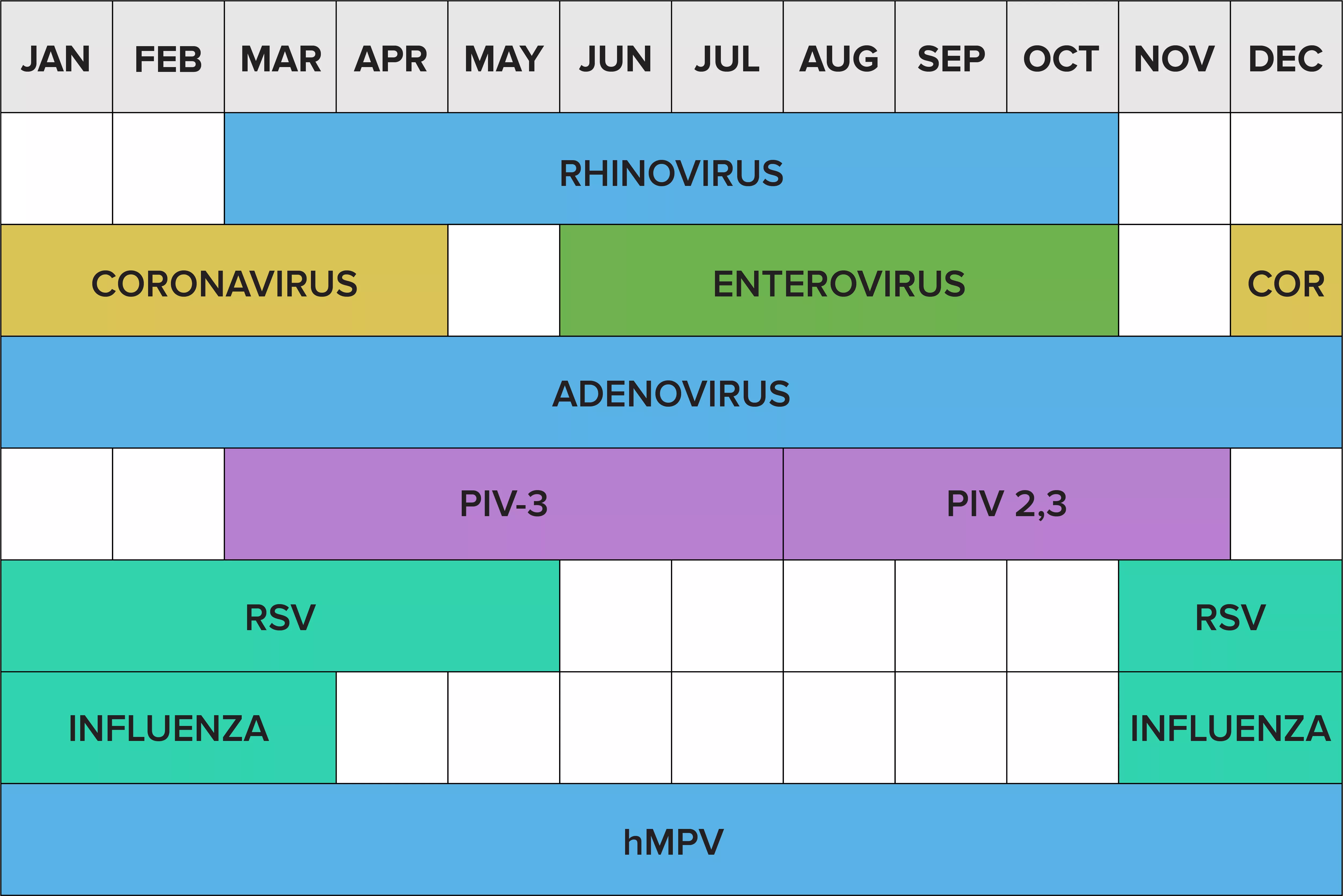
Note: Patterns for SARS-CoV-2 may continue to change in the coming years.
Patient symptoms don't translate to an easy diagnosis
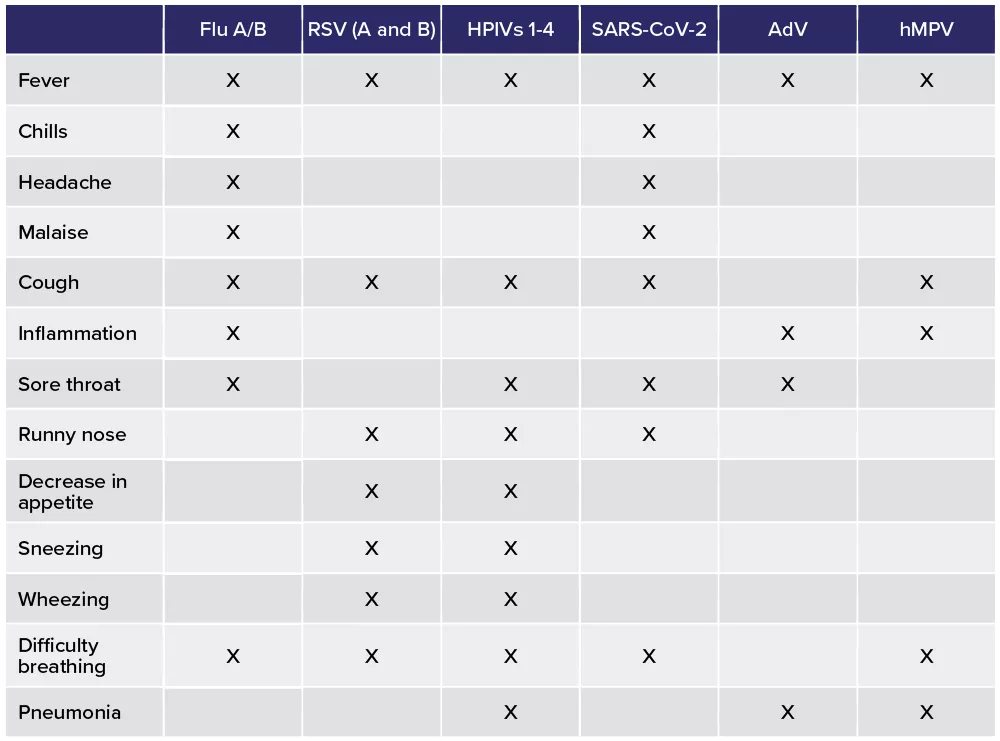
Identifying the viral cause of a respiratory disease based solely on patient symptoms can be difficult. Symptoms of respiratory infections due to different viruses often overlap, including commonly occurring ones like fever, malaise, cough and inflammation.5-10 This is without factoring in the common cold where symptoms can also include nasal congestion, sore throat, cough and fever most often caused by rhinoviruses.11
Table 1. Symptoms associated with common respiratory viruses.5-10
Accurate diagnosis has many benefits for patients, labs and the community
Accurate diagnosis allows for the initiation of appropriate treatment.11-12 Some respiratory viruses have very specific treatments, including influenza A/B, RSV, and SARS-CoV-2 which have the only FDA-approved treatments.13-15 Utilizing testing and differentiation may also help to avoid unnecessary treatments, as antibiotics should not be used to treat respiratory viral infections.16 Knowledge of patient viral infections can provide valuable information to hospitals and other medical facilities to help minimize the spread of viruses, as well as public health authorities who may be monitoring current viral spreads.17-20
Delivering an early, accurate diagnosis of the viral cause for respiratory infection is also important because some viruses can lead to the development of serious acute respiratory disorders. For example, many respiratory viral infections can lead to pneumonia, and other viruses, including influenza, RSV and rhinoviruses, can result in acute exacerbations of asthma and chronic obstructive pulmonary disease (COPD).5-7,9-10,21-23
Respiratory viruses can impact everyone, but certain patient groups, such as children and patients older than 65 years, can be especially hard hit. With RSV, 57,000 children under 5 years of age and 177,000 adults are hospitalized each year in the U.S.24 This virus also results in the death of 14,000 adults each year.24 There are also viruses that are highly prevalent in all patient groups, such as influenza, which has resulted in an estimated burden each year of up to 41 million illnesses, up to 710,000 hospitalizations and up to 52,000 deaths.25 These statistics highlight the importance of diagnosing with the right test and initiating treatment as early as possible to reduce mortality rates and help stem broader community infection.
Diagnosis based on viral infection symptoms is difficult, but fortunately there are highly sensitive and specific diagnostic technologies available, such as nucleic acid amplification tests (NAATs) that can help lead to accurate diagnoses of respiratory diseases without relying on diagnosis by symptoms alone.
Diagnostic stewardship: Test only for what you need
With the Aptima® and Panther Fusion® respiratory assays, labs can consolidate infectious disease testing, increase walkaway time and enhance testing flexibility. This can help labs control upfront testing costs, better align with reimbursement guidelines and decrease overall costs of care by eliminating further (and unnecessary) testing and inappropriate treatments.16 These assays deliver excellent clinical performance for viral detection and differentiation.26-30 With a wide array of mini-panel assays available, the Hologic Respiratory assays offer the flexibility to personalize patient testing and, from a single sample, test for specific viruses instead of using broad panels that may unnecessarily target 12-25 viruses at once.31,32 This flexibility offered by labs supports diagnostic stewardship and can help wth the management of healthcare costs without sacrificing care.32
Hologic offers these assays on the Panther® System to streamline the testing process and provide a fully automated workflow.32 This versatile system helps minimize waste regardless of whether one sample or over 1,000 samples per day are tested, while also allowing for the prioritization of urgent samples.33-35 With its ability to handle high volumes and sample prioritization, the Panther System provides the best of both worlds.
With respiratory viruses circulating year-round, accurate diagnosis is paramount
Diagnosing a respiratory infection based solely on symptoms is difficult because not only can several viruses be peaking simultaneously, but they may also have overlapping symptoms. With the assays and testing platform manufactured by Hologic, labs can offer the flexibility to test for subsets of viruses based on the time of year, local incidence and patient risk. Accurate diagnosis of respiratory infection helps determine the appropriate treatment for patients. It also helps to provide a more accurate snapshot of what viruses may be circulating within the community to inform public health countermeasures. Visit the Hologic Respiratory Solutions page for the diagnostic technologies available to help deliver accurate diagnoses.