AI Solutions Redefining Breast Care
Our world-class AI portfolio includes fully-featured, seamlessly integrated and evidence-backed AI solutions for powerful breast cancer detection.
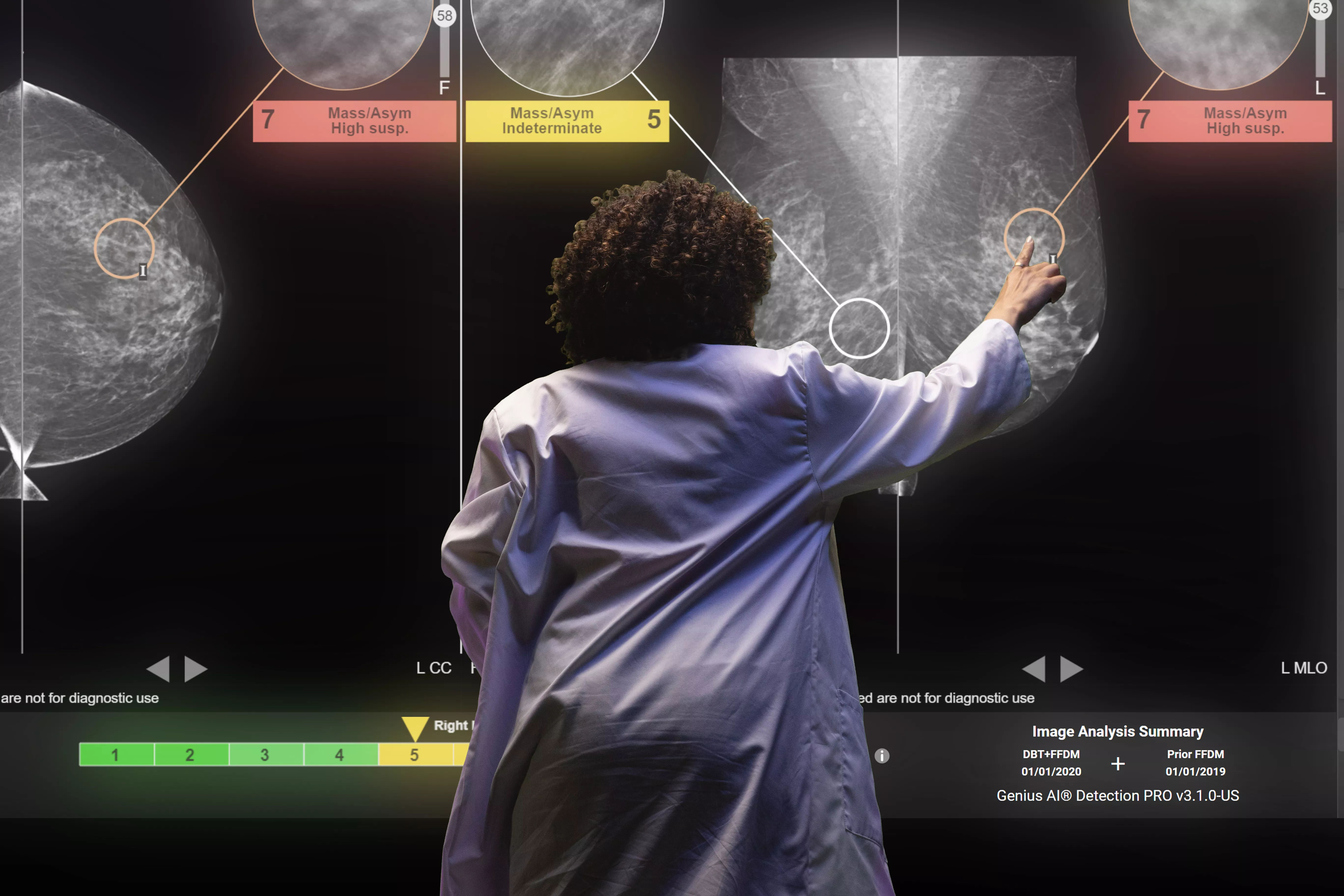
Industry-Leading Breast Cancer Detection AI for More Than 30 Years
We are pioneers in using AI to drive accuracy and efficiency for radiologists. Our AI technologies complement and elevate the Envision™ and 3Dimensions™ mammography systems, optimizing workflow at every step, from acquisition through diagnosis and beyond.
Hologic AI Breast Health Ecosystem
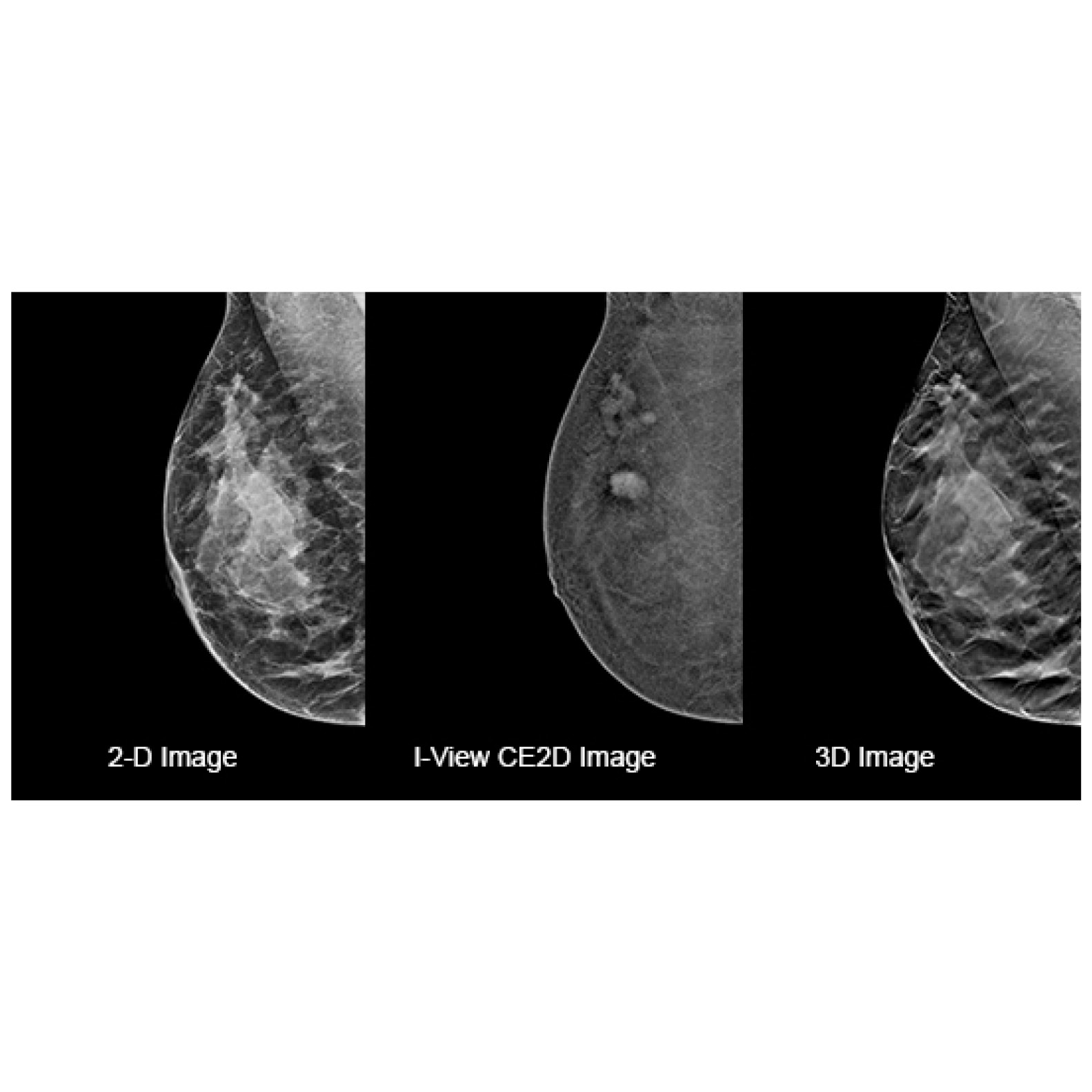
AI technology engineered for sophisticated, early breast cancer detection and smart image enhancements. Together, let's advance the standard of care.6

AI Breast Imaging Solutions Across the Full Patient Journey and Radiology Workflow
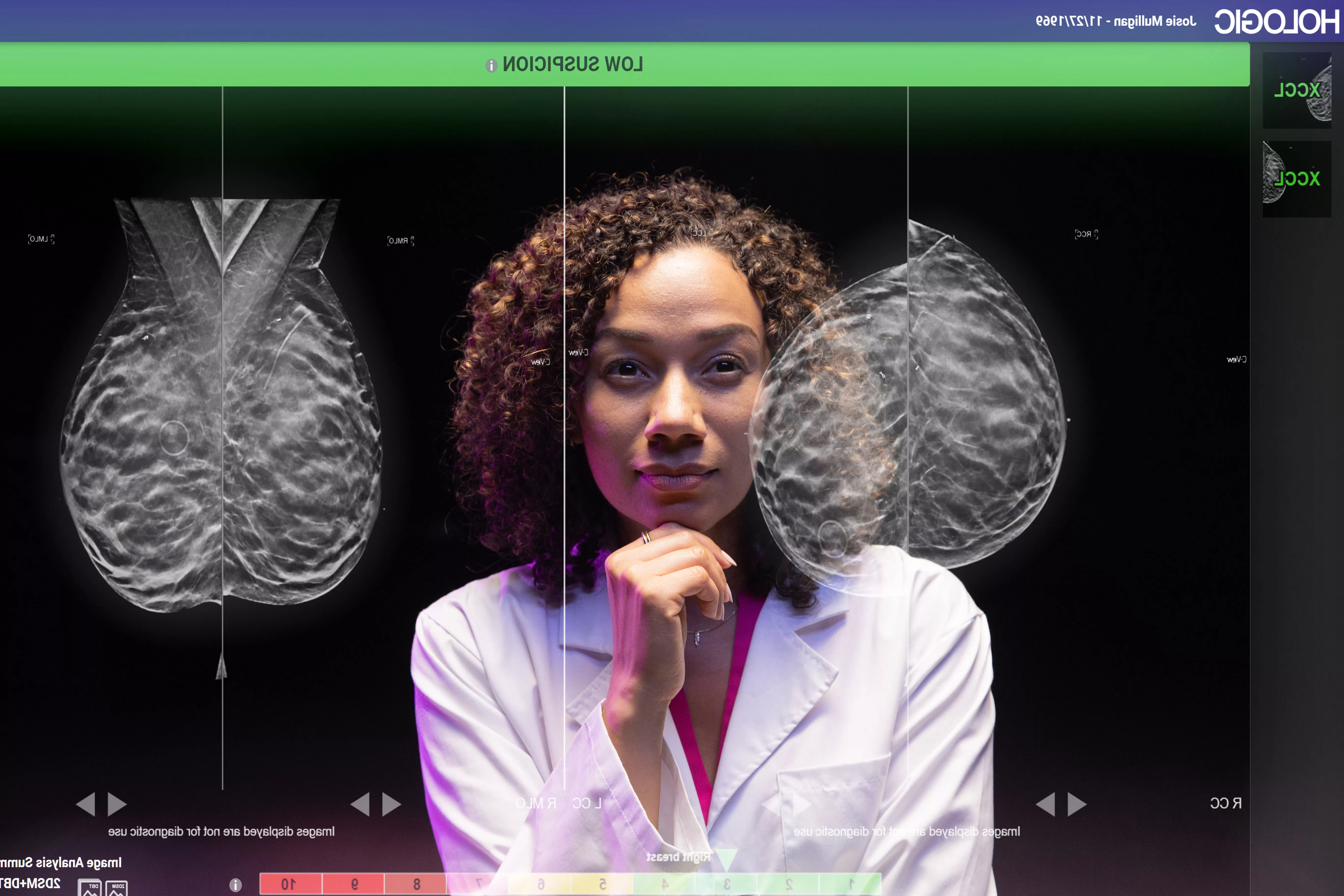
Harness the power of AI to better serve your patients
Give your patients total confidence in their care. Get meticulous readings with aided detection tools to boost precision, for fewer callbacks and fewer missed cancers.1,2,3 Tap into AI to set your facility apart with today’s top technology.
Genius AI® Detection PRO Assistant
Genius AI® Detection PRO solution breast imaging AI software utilizes the proven combination of 2D and DBT cancer detection AI, as well as the ability to aggregate prior exams, along with a comprehensive suite of tools that analyze, compare and score for efficient patient pre-reads.1
With automated lesion correlation, radiologists can be confident in understanding patient risks from the start of every appointment.2
All-Inclusive Cancer Detection Solution
Reviews And Reads Priors
Assess prior mammograms across patient history to better analyze lesion markings on individual cases.3
Provides A Breast Density Score
Utilize deep learning for accurate, unbiased breast density assessment with scoring consistency, helping to reduce inter-reading score variability, to inform individual screening plans.4
Clear Cut Case Scores
Know if it is suspicious or not with simplified color-coded case scoring from 1 – 10, reflect the highest lesion score found, for a reliable and easy to interpret reading experience.5
Automated Lesion Correlation
Sharpen your precision and reduce false positives4 and the need to search for correlates with automated lesion correlation marks between CC and MLO views.
A Full Set Of Powerful Tools In One Seamless Solution
A user-friendly interface displays all key clinical data, plus allows you to save time with automated pre-reporting that fill the worklist info and sends findings.
Integrated Artificial Intelligence Solutions
Our range of AI software solutions uses the latest technology to drive efficiencies and trust for your radiology department, facility and patients.
Hologic AI Solutions Insights from Industry Leaders
Visit Our Virtual Hospital
Browse our portfolio of Breast Health solutions in 3D. See how you can unlock the advantage of time across the entire Breast Continuum of Care.

* Compared to radiologists not using Genius AI Detection solution
1. Compared to not using Genius AI Detection PRO. 2. U.S. Food and Drug Administration, Center for Device and Radiological Health. (2020, November 18). Genius AI Detection K201019 510(k) Summary. IN1FDA Clearance: K201019 *Based on analyses that do not control type I error and therefore cannot be generalized to specific comparisons outside this particular study. In this study: The average observed AUC was 0.825 (95% CI: 0.783, 0.867) with CAD and 0.794 (95% CI: 0.748, 0.840) without CAD. The difference in observed AUC was +0.031 (95% CI: 0.012, 0.051). The average observed reader sensitivity for cancer cases was 75.9% with CAD and 66.8% without CAD. The difference in observed sensitivity was +9.0% (99% CI: 6.0%, 12.1%). The average observed recall rate for non-cancer cases was 25.8% with CAD and 23.4% without CAD. The observed difference in negative recall rate was +2.4% (99% CI: 0.7%, 4.2%). The average observed case read-time was 52.0s with CAD and 46.3s without CAD. The observed difference in read-time was 5.7s (95% CI: 4.9s to 6.4s). www.accessdata.fda.gov/cdrh_docs/pdf20/K201019.pdf. FDA clearance K221449 Make #1 2 and add FDA 510(k) Clearance K230096. 3.Kshirsagar, A. (2023). Comparison between ImageChecker CAD and GAID algorithm on sequestered FDA database. Refer to Hologic document (DHM-14593) 3. S. Pacilè, C. Aguilar, S. Chambon, and P. Fillard "Including temporal changes information to an AI system for breast cancer detection to reduce false positive rate", Proc. SPIE 12286, 16th International Workshop on Breast Imaging (IWBI2022), 122860O (13 July 2022); https://doi.org/10.1117/12.2624098 4. K240301 510(k) Summary Manufactured by Therapixel. Distributed by Hologic 5. S. Pacilè, et al. (2024). Evaluation of a multi-instant multi-modal AI system supporting interpretive and noninterpretive functions. Accepted for publication in the Journal of Breast Imaging, 6. U.S. Food and Drug Administration, Center for Device and Radiological Health. (2020, November 18). Genius AI Detection K201019 510(k) Summary. IN1FDA Clearance: K201019 *Based on analyses that do not control type I error and therefore cannot be generalized to specific comparisons outside this particular study. In this study: The average observed AUC was 0.825 (95% CI: 0.783, 0.867) with CAD and 0.794 (95% CI: 0.748, 0.840) without CAD. The difference in observed AUC was +0.031 (95% CI: 0.012, 0.051). The average observed reader sensitivity for cancer cases was 75.9% with CAD and 66.8% without CAD. The difference in observed sensitivity was +9.0% (99% CI: 6.0%, 12.1%). The average observed recall rate for non-cancer cases was 25.8% with CAD and 23.4% without CAD. The observed difference in negative recall rate was +2.4% (99% CI: 0.7%, 4.2%). The average observed case read-time was 52.0s with CAD and 46.3s without CAD. The observed difference in read-time was 5.7s (95% CI: 4.9s to 6.4s). www.accessdata.fda.gov/cdrh_docs/pdf20/K201019.pdf. FDA clearance K221449 Make #1 2 and add FDA 510(k) Clearance K230096. 7. U.S. Food and Drug Administration, Center for Device and Radiological Health. (2022, October 6). Genius AI Detection 2.0 K221449, K230096, 510(k) Summary. www.accessdata.fda.gov/cdrh_docs/pdf22/K221449.pdf 8. Tech File: TFL-00059 9. Report: CSR-00116 10. FDA approval number.P080003/S008 11. 510k K163623 https://doi.org/10.1093/jbi/wbae062.
Dr. Jason McKellop, Dr. Chad Reihm and Dr. James Schlund are paid consultants to Hologic. Views and opinions expressed herein by the medical professional are theirs alone and do not necessarily reflect those of Hologic.
Related Portfolio & Solutions

Breast Health Continuum of Care
Time is precious when it comes to effective detection, diagnosis and treatment of breast cancer. We strive to save you time at every step along the Breast Health Continuum of Care ensuring more women have more time in better health.

Breast Biopsy Solution
Explore Hologic's innovative solutions for breast biopsy procedures, designed for more reliable diagnosis and improved patient care. Learn about our cutting-edge technologies and products.
Breast Imaging
Discover Hologic's advanced breast screening and diagnostic solutions designed to improve early detection and women's health outcomes. Learn more about our products.
Breast Surgery Solution
Explore breast cancer surgery products from Hologic, including localization systems and wires, surgical markers and specimen imaging systems. The future of breast surgery is here.