Overview
Documents
Training
Simplified Processing.
Streamlined Review.
The ThinPrep® non-gynecological portfolio delivers high quality liquid-based cytology slides, with the uniform presentation and enhanced cellular detail required for accurate and efficient review.
Reliable Results
- ThinPrep® solutions enable optimal preservation and presentation for confident results
- An increase in sensitivity and specificity was found compared to conventional methods1
- Studies show a reduction in unsatisfactory cases compared to conventional methods2
Workflow Benefits
- One representative slide per case improves screening efficiency, saves storage space and reduces filing time
- Process GYN, Non-GYN, and UroCyte® samples from the same ThinPrep® processor
- Residual material provides a reproducible sample for use in ancillary testing
User Experience
- Smart automation reduces touch points and manual steps in slide preparation
- Less slides to review reduces time and energy, allowing cytologists and pathologists to focus on other tasks
- ThinPrep® technology offers a standardized and consistent process for all specimen types
Get started today with our ThinPrep® non-GYN consumables.

Enables optimal cellular preservation. ThinPrep® PreservCyt® solution is trusted by laboratories worldwide for maintaining specimen integrity and allowing for enhanced nuclear detail.

Standardizes urine collection for cytology and FISH testing. ThinPrep® UroCyte® PreservCyt® solution maintains specimen integrity. Also available in ThinPrep® UroCyte® Lab Validation and ThinPrep® UroCyte® Urine Collection kits.

Preserves cell morphology and reduces obscuring materials. ThinPrep® CytoLyt® solution lyses red blood cells, dissolves mucus, and prevents protein precipitation for a cleaner slide and clearer review.

Leverages thin-layer technology to produce an evenly distributed layer of cells. ThinPrep® Non Gynecological filters allow for controlled cell collection and transfer of non-gyn specimens. The ThinPrep® UroCyte® filter is uniquely equipped with a 10mm diameter filter area.

Provides a clean surface for cell transfer within a defined area. Labs can choose between ThinPrep® Non Gynecological and ThinPrep® Arcless microscope slides depending on their needs. For urine specimens, ThinPrep® UroCyte® microscope slides contain a 16mm deposition area to optimize reagent use.
The ThinPrep® workflow creates one representative slide per case
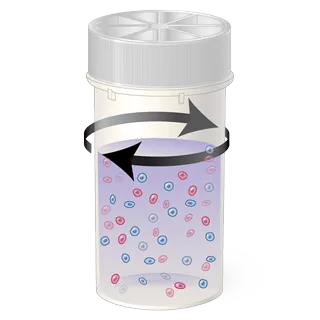
1. Cell Dispersion
The sample vial is rotated, separating debris and dispersing mucus gently enough to have no adverse effect on cell appearance.

2. Cell Collection
A vacuum is created within the filter, collecting cells on the surface of the membrane. Collection is controlled by the processor’s software, as it monitors the rate of flow through the filter.
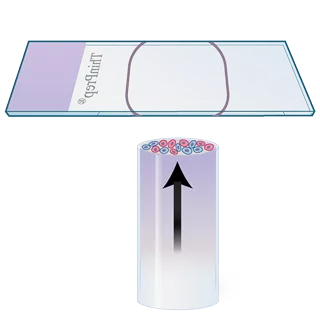
3. Cell Transfer
The filter is inverted and gently pressed against the glass slide. Natural attraction and slight positive air pressure cause the cells to adhere to the slide, resulting in even cell distribution within the defined area.
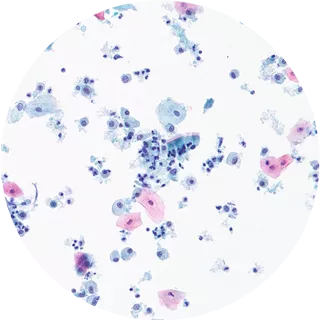
4. Clear Results
The resulting slide provides a clear representation of sample cells, free from obscuring elements for ease of visualization.
Bringing confidence and simplicity to cytology processing
Process GYN and Non-GYN samples on the same instrument. ThinPrep® processors are fully automated at every size, providing flexibility and efficiency to laboratories of all volumes.

A Clear View to Diagnostic Clarity
ThinPrep® technology provides clear cellular detail, regardless of specimen type.
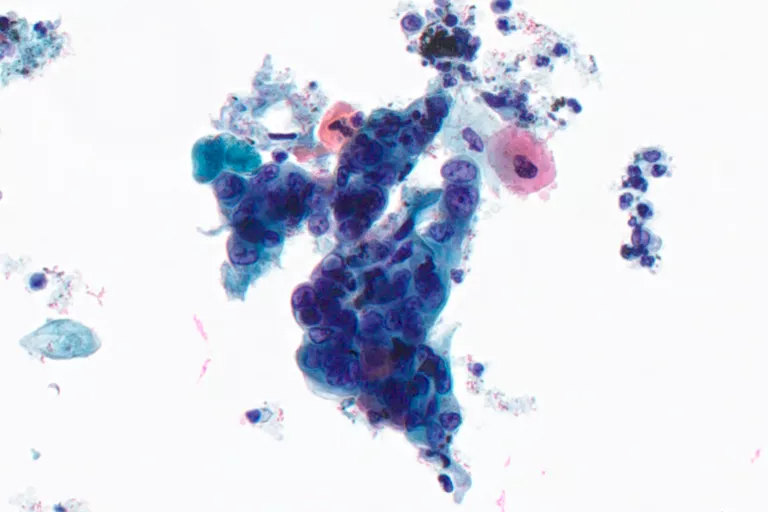
Fine Needle Aspirates
Thyroid, Lung, Lymph Node, Breast, Liver
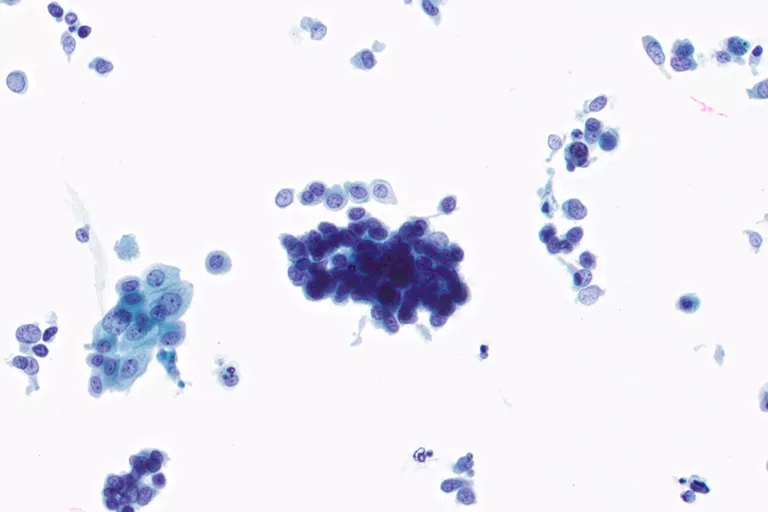
Body Fluids
Urine, Pleural Fluid, Ascitic Fluid, Pericardial Fluid, Cerebrospinal Fluid (CSF)
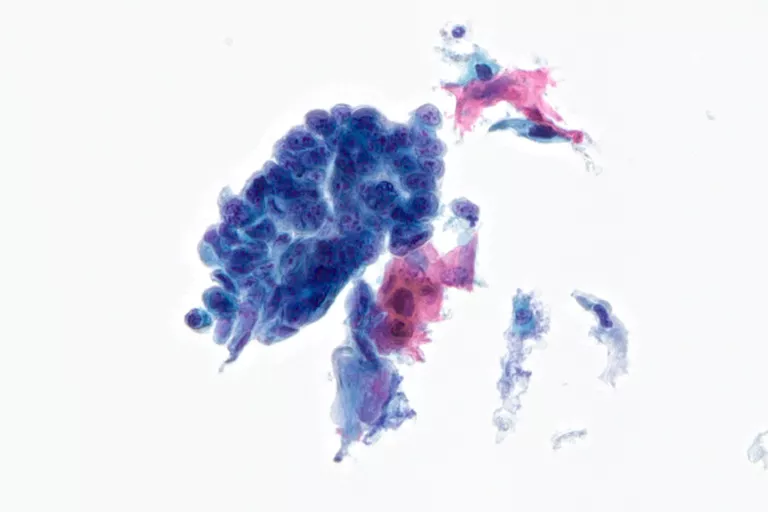
Mucoid
Bronchial Brush, Bronchial Wash, Bronchoalveolar Lavage, Sputum, Bile Duct Brush
Access the ThinPrep® Non-GYN Atlas and other educational resources at CytologyStuff.com

Pleural fluid mesothelioma cell block H&E
Pleural fluid mesothelioma cell block D2-40 Positive IHC
Residual material for ancillary testing
- Cell Blocks
- Special Staining
- Immunocytochemistry
- In Situ Hybridization
- DNA Ploidy Analysis
References:
1. Lee KR, et al: Evaluation of the ThinPrep Processor for Fine Needle Aspiration Specimen, Acta Cytol, 1996, Vol. 40(5):895-899 2. Fischler, DF, et al: Nongynecological Cytology Utilizing the ThinPrep Processor, Acta Cytol, 1996, Vol. 40:669-675
Safety Data Sheets
Package Inserts
Related Products
Overview
Package Inserts
Leading the charge in cervical disease prevention with the ThinPrep system and Aptima HPV assays
Hologic has remained an unwavering advocate for women’s health for more than two decades. Our goals as a company are intrinsically tied to changes in best-practices for women’s health, applying the latest findings in diagnostic medicine to the development of new products and technologies in response to the emergence of new discoveries in medicine.
Cervical disease screening is an essential component of our efforts in women’s health. Hologic is the leader in Pap and human papillomavirus (HPV) testing. The ThinPrep Pap test helps healthcare providers detect the presence of abnormal cervical cells, and the Aptima HPV assays identify high-risk HPV mRNA that is indicative of the HPV infections most likely to lead to cervical disease.1-3
Today, screening with Pap+HPV Together (co-testing) provides the best possible protection against cervical cancer for women ages 30-65.4-6 Now, what was once a top cancer among women is up to 93% preventable.7
The introduction of the Pap smear and, later the ThinPrep Pap test, have contributed to a decline in cervical cancer rates of more than 60% since the 1950s.1,8 Since then, HPV has been identified as a cause of cervical cancer, and HPV testing and vaccinations have been developed.9
These medical triumphs have fortified the medical community’s ability to detect and prevent cervical disease and cancer. Today, the combination of data-supported guidelines for cervical cancer screening and the availability of HPV vaccination are key in the fight for women’s health.
Hologic released the first liquid-based cytology option in cervical disease screening in 1996: the ThinPrep Pap test.1 Today, more than 20 years after the release of the ThinPrep Pap test, it remains the preferred choice in Pap testing in the United States.10 ThinPrep Pap tests account for more than 80% of Pap tests performed in the United States, with 650 million tests performed globally so far.10
More than 170 scientific studies involving the ThinPrep Pap test have demonstrated its benefits, including increased disease detection, reduction of equivocal diagnoses, improved specimen adequacy, adjunctive molecular testing and morphology assessment.11
The College of American Pathologists reported increased HSIL (high-grade squamous intraepithelial lesions) and LSIL (low-grade squamous intraepithelial lesions) in labs using LBC versus labs that utilized conventional Pap testing.12 It also showed increased sensitivity for cervical adenocarcinoma over conventional Pap testing.13
The Aptima HPV assay and Aptima HPV 16 18/45 genotype assay target HPV types that pose the largest threat to women.2,3 While other HPV assays target DNA, the Aptima HPV assays target mRNA, which studies show reflects the presence and activity of high-risk HPV infection.2,3 The Aptima HPV assay detects E6/E7 mRNA, which is indicative of those HPV infections more likely to cause cervical disease.2 The Aptima HPV 16 18/45 genotype assay identifies HPV types 16, 18 and 45, which are associated with up to 80% of all invasive cervical cancers worldwide.3,14-15
Screening women with Aptima HPV assays helps providers optimize care for each patient.
The ThinPrep Pap test is just one of several offerings in the ThinPrep system. The ThinPrep processors, imagers and review scopes help improve workflow in laboratories and assist with cytotechnologists’ ability to identify abnormalities. These instruments help complete the ThinPrep system and maximize laboratory efficiency and accuracy in disease detection.
In addition to the ThinPrep Pap test, Hologic remains committed to providing laboratories with innovative and effective cytology solutions for non-gynecological testing needs.
Processors
Imagers and Review Scopes
Combines the power of ThinPrep imaging and manual microscope review in a single desktop system, with slides imaged and ready for review in approximately 90 seconds.
Identifies 22 areas of interest within the ThinPrep Pap test slides with the largest, darkest nuclei for cytotechnologists to examine.
Same trusted algorithm as the ThinPrep image processor, but with a faster camera, expanded label-reading formats and on-demand imaging.
Presents the 22 fields of view to the cytotechnologist for their review.
Combines the power of the review scope with the flexibility of a manual microscope.
Stainers
Now Available Compass Stainer: Adds flexibility to both routine and special staining procedures. Comes programmed with the ThinPrep Stain Protocol for customer convenience.
From Pap and HPV testing to our full range of diagnostic products and cross-divisional innovations like the Genius™ 3D Mammography exam for breast health, Hologic delivers for women. Our promise to offer innovative products to meet the needs of women globally persists throughout all that we do, including in cervical disease screening. Working together, we can achieve the goal of defeating cervical disease.