Aptima® Vaginal Health
Deliver accurate results with the Aptima® BV and Aptima® CV/TV assays.
Overview
Documents
Training
Vaginitis is a leading reason for OB/GYN visits.1-3
Most women have symptoms of vaginitis at some point during their lives. The prevalence in the United States is estimated to be 21.2 million (29.2%) among women ages 14-49.2 The vast majority (90%) of vaginitis is caused by bacterial vaginosis (BV), Candida vaginitis (CV) (commonly referred to as a yeast infection), or Trichomonas vaginalis (TV)—either individually or a combination of all three infections.1,4 Overlapping symptoms and co-infections make clinical diagnosis a challenge, and 30% of symptomatic women will go undiagnosed after clinical evaluation.5-8
Aptima Assays Deliver Highly Sensitive Quantitative Detection Across Multiple Targets
With the prevalence of co-infections and similarity in symptoms, it is important to determine the underlying cause of vaginitis. Our assays:
Deliver Objective Results
CDC- and ACOG-recommended nucleic acid amplification tests (NAATs) deliver results that are more comprehensive and higher in sensitivity and specificity versus DNA probe and traditional clinical methods5, 9-11
Detect More Co‑infections
Molecular NAATs can detect 3x more mixed infections than clinical diagnosis with wet mount, culture and Amsel’s criteria10
Help Improve Quality of Life
Vaginitis can negatively impact a woman’s quality of life, particularly in those with recurrent symptoms. Proper diagnosis and treatment can reduce anxiety and discomfort.4
Detect with Certainty
The FDA-cleared Aptima assays run on the Panther® system and provide easy-to-interpret qualitative results from highly sensitive quantitative detection.
Aptima BV Assay13
- Unlike other tests that only detect one BV agent, the Aptima BV assay targets: Lactobacillus, G. vaginalis and A. vaginae
- The Aptima BV assay utilizes an advanced proprietary algorithm to report a simple positive or negative result
- The assay detects the presence of BV-associated agents
- The Aptima BV assay provides a clear diagnosis for BV with a high sensitivity of 95-97.3% and specificity of 85.8-89.6%
Aptima CV/TV Assay14,15
- The Aptima CV/TV assay reports the Candida species group (C. albicans, C. tropicalis, C. parapsilosis and C. dubliniensis), Candida glabrata and TV to deliver 3 positive or negative results
- The Aptima CV/TV assay differentiates C. glabrata from the Candida species group
- Sensitivity for CV is 84.7-92.9% with a specificity of 91.0-99.1%
- Sensitivity for TV is 96.5% with a specificity of 95.1-98.9%
Risks of Delayed Diagnosis or Misdiagnosis
Untreated vaginitis infections can be associated with an increased risk of:

Endometritis15
Pelvic inflammatory disease (PID)15
Sexually transmitted infections (STIs) and human immunodeficiency virus type 1 (HIV-1)16-18
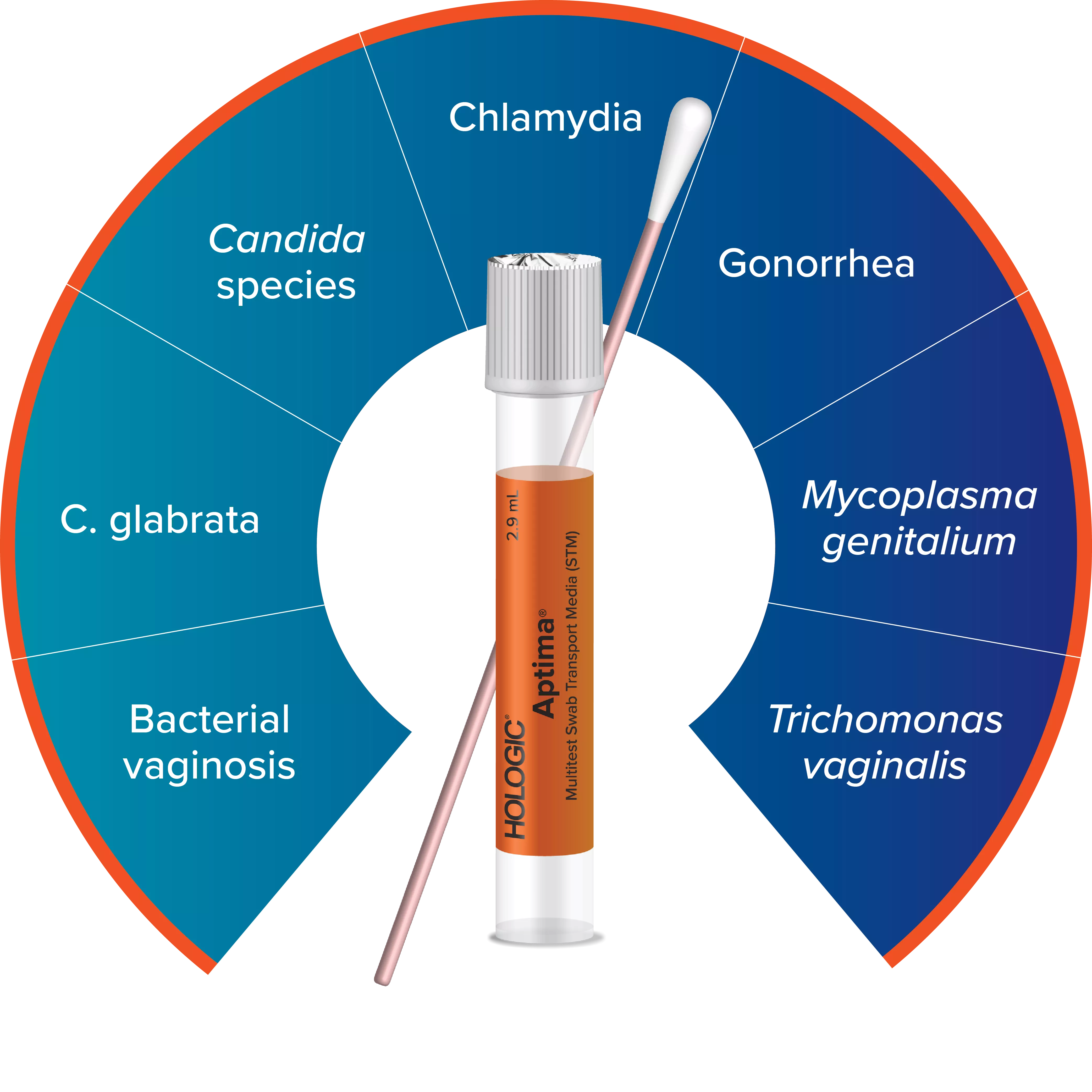
Expand Your Offerings with the Aptima® Multitest Swab
Using the Aptima Multitest Swab, one vaginal swab sample can be collected to test for BV, CV, and TV.13-14, 19-21
The Latest on Aptima Vaginal Health
1. Kent HL. Epidemiology of Vaginitis. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 2):1168-76. 2. Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, Markowitz LE. The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007 Nov;34(11):864-9. 3. Van Der Pol B. 2010. Diagnosing vaginal infections: It’s time to join the 21st century. Curr346 Infect Dis Rep 12:225-230. 4. Paladine HL. Vaginitis: Diagnosis and Treatment. Am Fam Physician. Am Fam Physician. 2018 Mar 1;97(5):321-329. 5. Anderson MR, Klink K and Cohrssen A. Evaluation of Vaginal Complaints. JAMA. 2004;291(11):1368–1379. doi:10.1001/jama.291.11.1368. 6. Miller JM, Binnicker MJ, Campbell S, et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67(6):e1-e94. 7. Wiesenfeld HC, Macio I. 1999. The infrequent use of office-based diagnostic tests for vaginitis. Am J Obstet Gynecol 181:39–41. 8. Hainer BL, Gibson MV. Vaginitis. Am Fam Physician. 2011;83(7):807-815. 9. Workowski, et al. Sexually Transmitted Infections Treatment Guidelines 2021. MMWR Recomm Rep 2021;7. 10. Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56. doi:10.1128/JCM.00252. 11. ACOG Practice Bulletin No. 72. Clinical management guidelines for obstetrician-gynecologists: Vaginitis. Obstet Gynecol reaffirmed 2019; 107(5): 1195-1206. 12. Caza M, Marthe C, Locher K, Hoang L, Tucker M, Mandy J, Jewsbury H, Wilmer, Amanda. Evaluation of the Aptima BV and CV/TV assays compared to conventional laboratory based testing methods for the diagnosis of vaginitis, Diagnostic Microbiology and Infectious Disease. Diagnostic Microbiology and Infectious Disease. 2023, Apr. Volume 106, Issue 4. 13. Aptima BV Assay Package Insert. AW- 23712 Rev. 001. San Diego, CA: Hologic, Inc, 2019. 14. Aptima CV/TV Assay Package Insert. AW-18812 Rev. 001. San Diego, CA: Hologic, Inc, 2019. 15. Haggerty CL, Hillier SL, Bass DC, Ness RB; PID Evaluation and Clinical Health study investigators. Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin Infect Dis. 2004 Oct 1;39(7):990-5. Epub 2004 Sep 2. 16. Bautista CT, Wurapa EK, Sateren WB, Morris SM, Hollingsworth BP, Sanchez JL. Association of Bacterial Vaginosis with Chlamydia and Gonorrhea Among Women in the U.S. Army. Am J Prev Med. 2017;52(5):632-639. doi: 10.1016/j.amepre.2016.09.016. 17. Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003 Aug 1;37(3):319-325. 18. Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9(6):e1001251. 19. Aptima Trichomonas vaginalis Assay [package insert]. AW-27552, San Diego, CA; Hologic, Inc., 2020. 20. Aptima Mycoplasma genitalium assay [package insert]. AW-17946, San Diego, CA; Hologic, Inc., 2022. 21. Aptima Combo 2 Assay [package insert]. AW-20535, San Diego, CA; Hologic, Inc., 2021.
Documents
Aptima Vaginal Health Flashcard
Safety Data Sheets
Package Inserts
Related Products
Overview
Documents
Aptima Vaginal Panel
The Aptima® vaginal panel from Hologic consists of molecular nucleic acid amplification tests (NAAT) to aid in the detection of vaginosis and vaginitis. Our assays detect the three most common causes of infectious vaginitis: bacterial vaginosis, candida vaginitis and trichomoniasis.
Vaginitis is a common problem that affects millions of women
Ten million women each year visit their healthcare providers seeking a cure for vaginitis and a third of all women will have symptoms of vaginitis at some point during their lives, most commonly during their reproductive years.1,2 Some types of vaginitis are transferred through sexual activity, while others arise because of an imbalance in the bacterial or fungal makeup of the vaginal microbiome.3 Combined, bacterial vaginosis (BV), candida vaginitis (commonly known as a yeast infection, CV), and trichomoniasis (Trichomonas vaginalis, TV) make up 90% of vaginitis cases.3
Hologic is an innovative medical technology company focused on improving women’s health, and features two assays that aid in diagnosing BV, yeast infections, and trichomoniasis. Accurate diagnosis using the Aptima vaginal panel supports better guided drug treatment, fewer recurrent doctor’s office visits, and reduced suffering in affected women. The Aptima® BV and Aptima® CV/TV assays* usher in a new era of molecular testing, offering a comprehensive and accurate diagnosis of vaginosis and vaginitis.
*Aptima® CV/TV assays is not available in Canada at this time. Please check with your sales rep.
Establishing a new standard for diagnosing vaginitis
The Aptima BV and Aptima CV/TV assays are indicated for use in symptomatic women to aid in the detection of BV, Candida species, Candida glabrata, and Trichomonas vaginalis on the Panther® system. Specimens must be collected with the familiar orange Aptima® Multitest Swab Specimen Collection Kit, via either clinician-collected or patient-collected vaginal swabs. As with our other Aptima diagnostic assays, one vaginal swab specimen can yield multiple test results on the automated Panther® system.
Bacterial Vaginosis
The prevalence of bacterial vaginosis or “BV” in the United States is estimated at 21 million among women ages 14–49.6 Healthcare practitioners often overlook the association of untreated BV infections with serious consequences, including pelvic inflammatory disease, cervicitis, higher risk of acquiring STIs (chlamydia, gonorrhea, HSV, HIV), spontaneous abortion, and preterm birth.7-13 The Aptima BV assay demonstrates excellent sensitivity and specificity, due to its proprietary algorithm and assay design, targeting Gardnerella vaginalis, Atopobium vaginae, and Lactobacillus species. A simple qualitative BV positive or negative result is returned.

Candida Vaginitis and Trichomonas Vaginalis
Commonly known as a yeast infection, vulvovaginal candidiasis (candida vaginitis, "CV") is a result of an overgrowth of fungal organisms, usually Candida albicans. Yeast infections can also be caused by the azole-resistant strain Candida glabrata, which is prevalent 8-16% of the time and requires a different treatment pathway than C. albicans.14,15 The Aptima CV/TV assay differentiates between Candida species and C. glabrata and can help healthcare providers determine the most appropriate antifungal therapy for their patients.
Sometimes referred to as "trich", TV is the most common curable STI in the United States.16 Left untreated, TV infection is associated with an increased risk of HIV acquisition and transmission, prolonged HPV infection, higher risk of acquiring STIs such as chlamydia, gonorrhea and HPV, and can lead to premature labor and low birth weight babies.17-21 The CDC recommends testing for TV in all women seeking treatment for vaginal discharge.22 The Aptima CV/TV assay meets the recommendations by the CDC for a highly sensitive and specific test for detecting TV.
One orange tube, one solution, maximum efficiency
The Aptima vaginal panel rounds out Hologic’s Aptima assay portfolio for sexual and vaginal health, which now includes vaginitis testing alongside comprehensive STI and viral load testing. Labs and healthcare providers now have the flexibility to detect up to seven infections and disease states with just one vaginal swab, including BV, Candida species, C. glabrata, Trichomonas vaginalis, chlamydia, gonorrhea, and Mycoplasma genitalium.23