Overview
Documents
Training
Empower Your Genius With Ours
The Genius® Digital Diagnostics System is the first and only FDA-cleared digital cytology system—combining a new artificial intelligence (AI) with advanced volumetric imaging technology to help cytologists and pathologists identify precancerous lesions and cervical cancer cells.
The Genius Digital Diagnostics System can help laboratories increase disease detection, enhance screening efficiency, and optimize workflows and resources. 1-6
The Genius Digital Diagnostics System provides more sensitive detection than manual and image-guided review.1
7.5%
increase in HSIL+ sensitivity vs. manual review1
3.6%
increase in HSIL+ sensitivity vs. ThinPrep® Imaging System review1
6.2%
Increase in endocervical detection compared to ThinPrep® Imaging System review1
5.3%
Increase in infectious organism detection compared to manual ThinPrep review1
Independent studies show higher screening efficiencies with Genius® Cervical AI review, with approximately 50% less time needed to review each case.1-3
Unlock a New Level of Operational Efficiency
AI-guided cytology increases workload potential with 0.5 slide equivalent review, and studies show approximately 50% less review time per case.1-4,6*
A streamlined digital workflow and increased screening efficiencies can help laboratories optimize resources and direct them to where they are needed most.4
More efficient workflows and review could result in capacity expansion and growth opportunities for the laboratory, such as volume increases and new business potential.
First-in, first-out imaging provides cases in order, review efficiencies help decrease time spent per case, and a digital workflow reduces slide movement and wait time.5,6
Case digitization and remote review helps reduce cumbersome glass slide management, archive requests, and locating lost or fixing broken slides to streamline workflows.4,6
Transform screening with artificial intelligence, advanced imaging, and digital efficiency.
Video Resources
1. Genius Digital Diagnostics System with Genius Cervical AI algorithm. US Instructions for Use AW-23890-001. Hologic, Inc.; 2024. 2. Ikenberg, H., Lieder, S., Ahr, A., Wilhelm, M., Schön, C., & Xhaja, A. (2023). Comparison of the Hologic Genius™ Digital Diagnostics System with the ThinPrep® Imaging System—a retrospective assessment. Cancer Cytopathology, 131(7), 424–432. https://doi.org/10.1002/cncy.22695. 3. Cantley, R. L., Jing, X., Smola, B., Hao, W., Harrington, S., & Pantanowitz, L. (2024). Validation of AI-assisted ThinPrep® Pap test screening using the Genius Digital Diagnostics System. Journal of Pathology Informatics, 15, 100391. https:// doi.org/10.1016/j.jpi.2024.100391. 4. Doxtader, E. E., et al. (2025). Papanicolaou test interpretation utilizing the Hologic Genius™ Digital Diagnostics System vs. manual glass slide review: A retrospective study of 596 cases. American Journal of Clinical Pathology. https://doi.org/10.1093/ajcp/aqaf080. 5. Harinath, L., Elishaev, E., Ye, Y., Matsko, J., Colaizzi, A., Wharton, S., Bhargava, R., Pantanowitz, L., & Zhao, C. (2024). Analysis of the sensitivity of high-grade squamous intraepithelial lesion PAP diagnosis and interobserver variability with the hologic genius digital diagnostics system. Cancer Cytopathology, 133(1). https://doi.org/10.1002/cncy.22918. 6. Murphy, K. M., et al. (2025). Impact of the Genius Digital Diagnostics System on workflow and accuracy compared with the ThinPrep Imaging System for review of ThinPrep Papanicolaou tests. American Journal of Clinical Pathology. https:// doi.org/10.1093/ajcp/aqaf099.
Documents
Safety Data Sheets
Package Inserts
Supporting Documentation
Related Products
Hologic introduces the Genius Digital Diagnostics System*
Genius Digital Diagnostics is the first CE-marked digital cytology platform to combine a new artificial intelligence (AI) algorithm with advanced volumetric imaging technology to help cytotechnologists and pathologists identify pre-cancerous lesions and cancer cells in women.
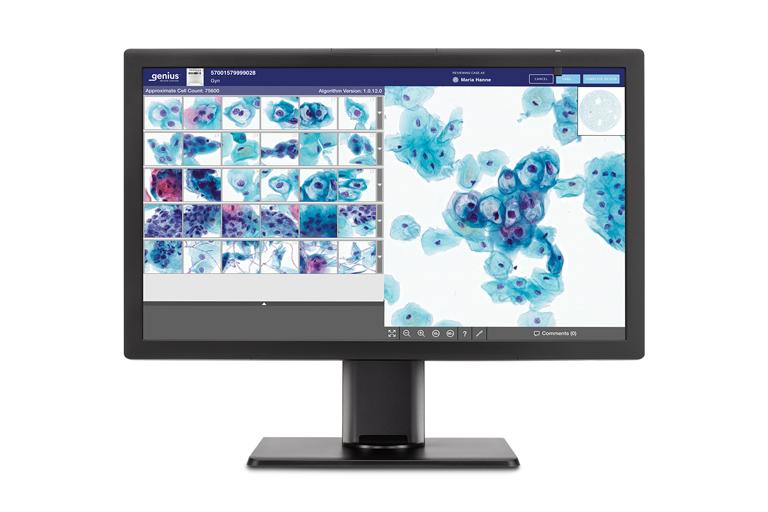
The system can rapidly analyze all cells on a ThinPrep® Pap test digital image, narrowing tens of thousands of cells down to an AI-generated gallery of the most diagnostically relevant images. This will help provide healthcare providers with the critical information they need to guide earlier detection and better treatment decisions for the patients they serve.
* Genius Digital Diagnostics is CE-marked for diagnostic use in Europe. May not be available in all markets. Contact your local Hologic representative for availability in your country.
The only comprehensive cervical cancer screening portfolio from sample collection to digital diagnosis.
The Genius Digital Diagnostics System’s cutting-edge technology includes:
Image Clarity
An advanced digital imager featuring volumetric imaging technology that produces exceptional image clarity.
Secure Image Management
A secure image management server for storing images.
New AI Algorithm
A new, deep learning-based artificial intelligence algorithm that is designed to detect pre-cancerous lesions and cervical cancer cells.
Review Station
A high-resolution review station for local or remote case review.
Explore the system
Genius Digital Diagnostics enables seamless and dynamic collaboration across laboratories within a network, connecting pathologists with remote review so each patient can benefit from the collective knowledge of geographically dispersed experts when needed. Digital case review promises to enhance the experience for lab partners by improving workflow and accelerating review time.