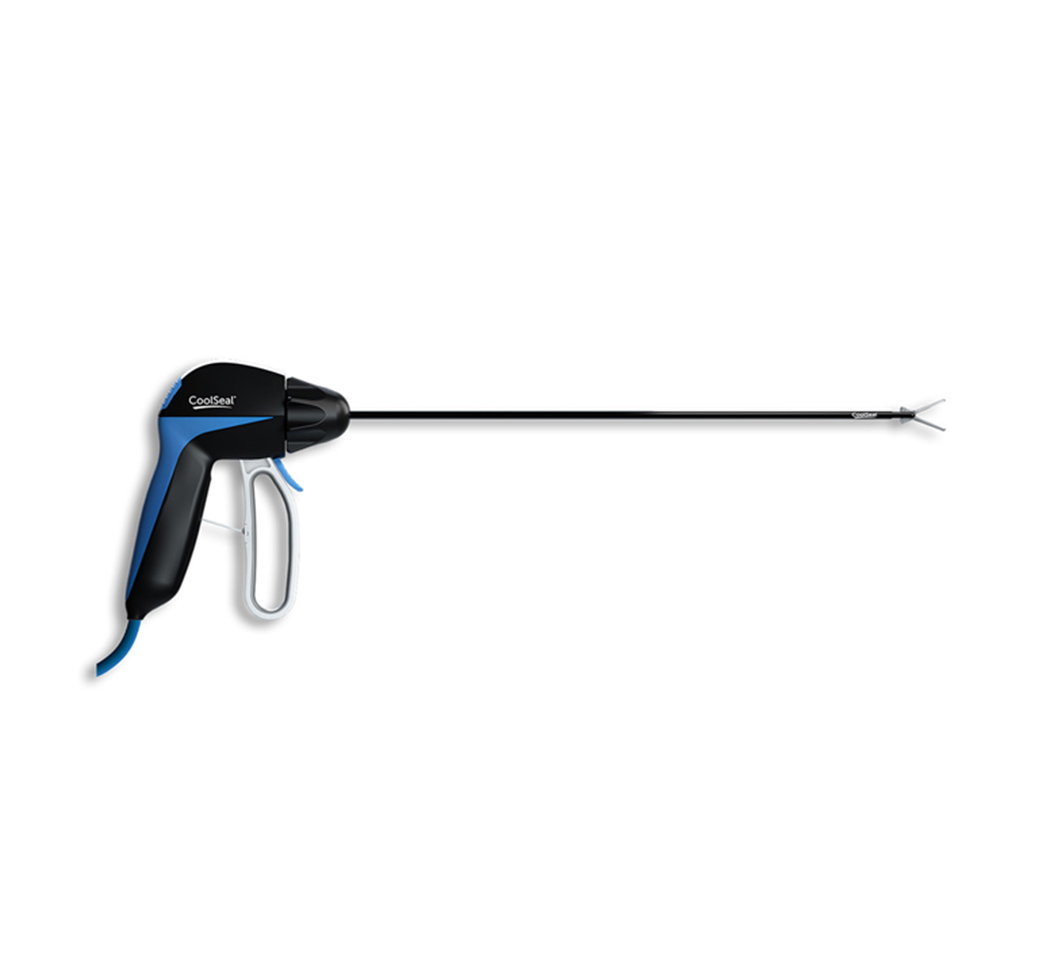
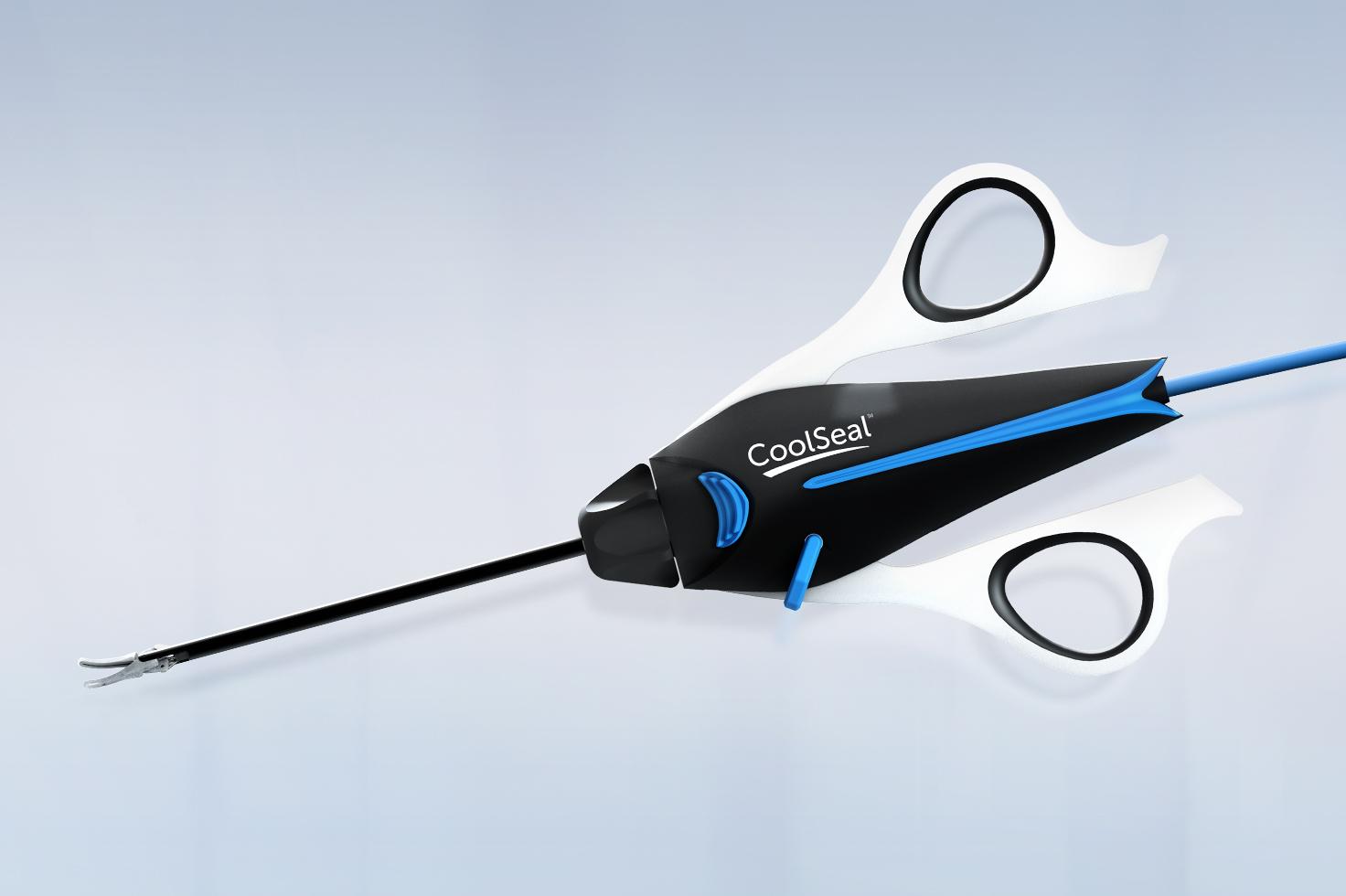
CoolSeal® Reveal Vessel Sealer
A sealer, divider and dissector tailor-made for precision and safety around critical structures in open procedures.
Overview
Documents
Training
Surgical Precision & Safety
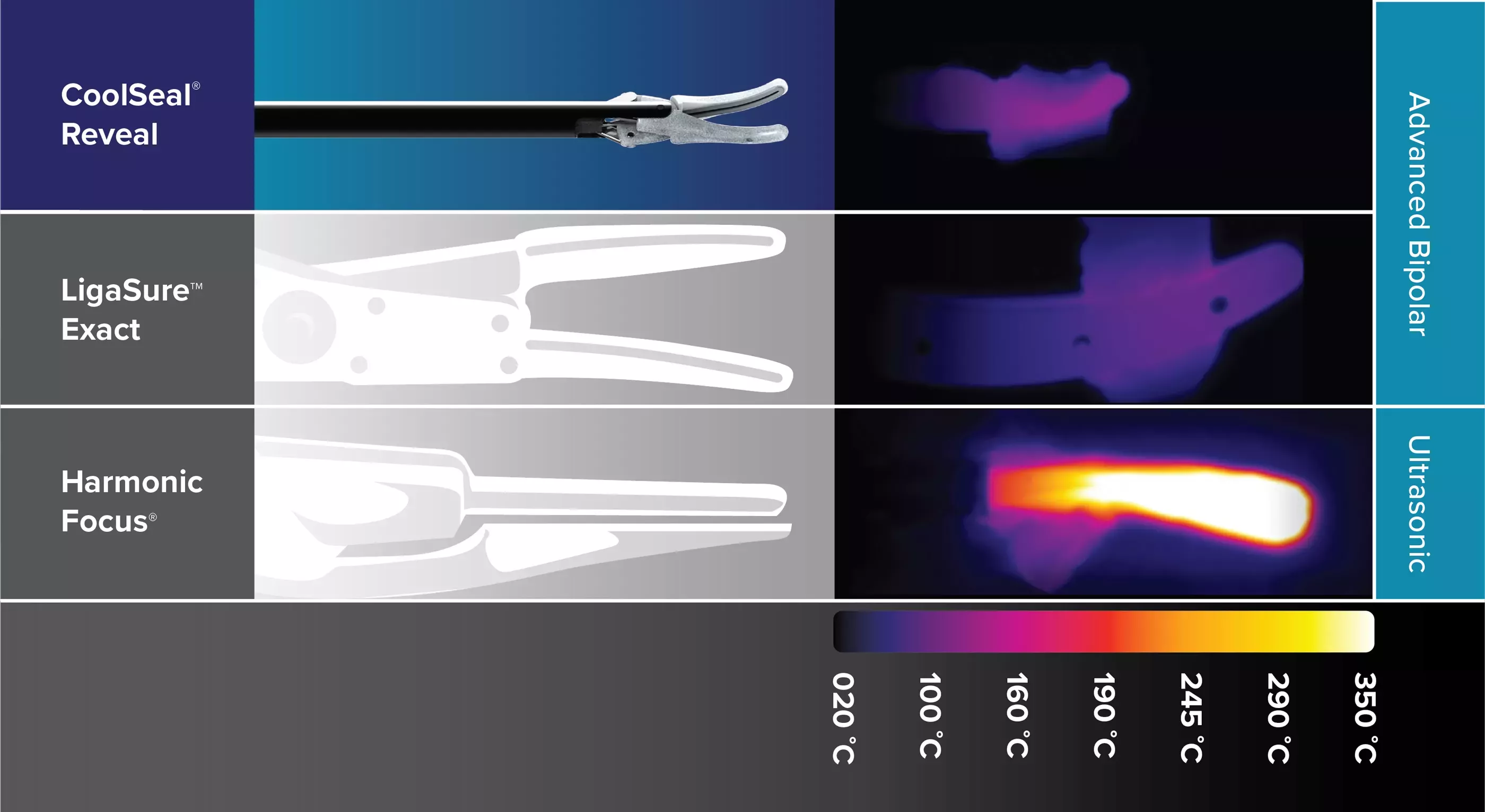
CoolSeal Reveal device is the only open, fine jaw advanced bipolar vessel sealer with a shafted design to maximize visibility in tight spaces.1 Minimize the thermal footprint near critical structures with outer jaws that stay cool throughout the procedure.2
Minimal Thermal Spread
Increase safety around critical structures with devices that feature <1mm thermal spread4 on average and jaws that rapidly cool after sealing.5
Fast Seals
Produce consistently strong, secure seals4 in less than 1 second on average.5
Deep Dissection
Maximize access and visibility with a shafted design and slim, dual-action jaws for deep dissection.

Proprietary Vessel Sealing Algorithm
CoolSeal technology is differentiated by a proprietary, low thermal profile algorithm that delivers precisely the power needed to seal vessels7 and discontinues when the seal is complete.
Cool.5 Fast.6 Slim.1

Specialties
Adult & Pediatrics: General, Urology, Thoracic, Plastic, Breast
Adult Only: Endocrine, Head & Neck / ENT
Procedures
Thyroidectomy, Parathyroidectomy, Radical neck dissection, Parotidectomy, Sentinel Node Biopsy, Hemorrhoidectomy
Maximize Visualization6
CoolSeal Reveal jaws are 46% narrower than LigaSure Exact jaws.6
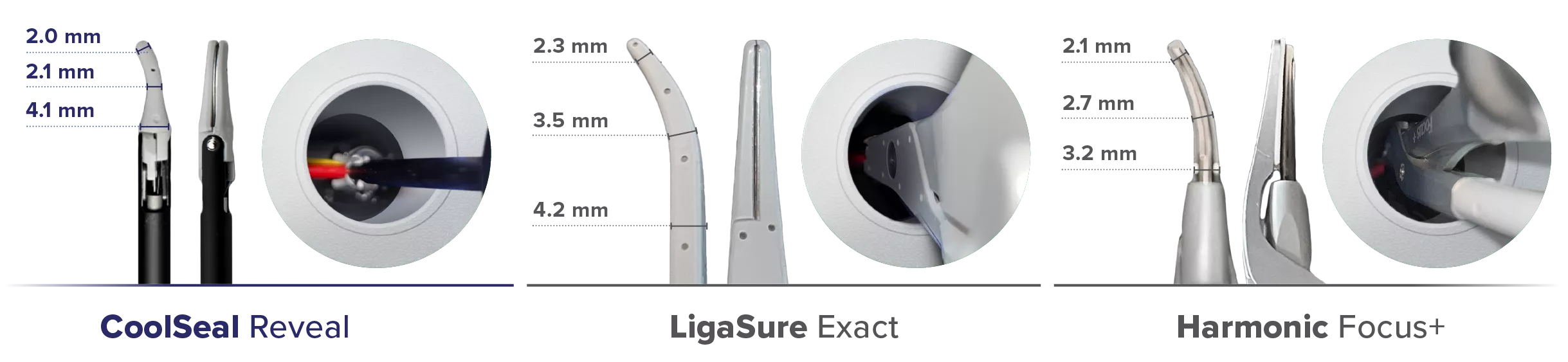
Thermal Profile Comparison
Vizient Award
The CoolSeal® advanced energy portfolio and JustRight® 5mm stapler were awarded the Innovative Technology designation from Vizient, Inc., the nation’s largest provider-driven healthcare performance improvement company. Vizient awarded the contract after hospital experts who served on one of its provider-led councils recommended CoolSeal and JustRight.
These provider-led councils evaluate products submitted through Vizient’s Innovative Technology Program. They recommend contracts for technologies that have the potential to enhance clinical care, patient safety and healthcare worker safety, or to improve business operations of healthcare organizations.
View the Hologic press release.
1. 16503 Rev 1 Reveal Pediatric Claims Report 2. 16457 Rev 1 CoolSeal™ Reveal Seal Plate Cooldown Comparison Marketing Report Average cooling time following a single activation 3. 16457 Rev 1 CoolSeal™ Reveal Seal Plate Cooldown Comparison Marketing Report Average cooling time following a single activation 4. 16339 Rev 1 CoolSeal Reveal Seal Quality Comparison Design Verification Report 5. 16451 Rev 1 CoolSeal Reveal Chronic Study Seal Times and Vessel Count Engineering Report 6. 16504 Rev 1 Reveal Jaw Size Claims Report (After CoolSeal reveal Jaws are 46% narrower than LisaSure Exact Jaws)
Related Products
Safety Data Sheets
Package Inserts

Overview
Package Inserts
Resources
Syndromic Respiratory Testing.
Patient-Specific Results.
The Panther Fusion Respiratory assays are the premier set of assays on the Panther Fusion® system. You can provide truly personalized syndromic respiratory testing with qualitative detection and differentiation of the most common respiratory viruses from a single patient sample. Each Panther Fusion Respiratory assay can be processed independently or simultaneously with other Panther Fusion® and Aptima® assays.
The Panther Fusion® SARS-CoV-2/Flu A/B/RSV, Panther Fusion® Flu A/B/RSV, Panther Fusion® Paraflu, Panther Fusion® AdV/hMPV/RV and Panther Fusion® Bordetella assays comprise the CE-IVD respiratory testing menu on the fully automated Panther Fusion system. Each is a multiplex, real-time PCR in vitro diagnostic test. These assays can be run on nasopharyngeal (NP) swab specimens obtained from individuals exhibiting signs and symptoms of a respiratory tract infection.1-5 Additionally, the Panther Fusion® SARS-CoV-2 assay has received Emergency Use Authorization for the detection of SARS-CoV-2 from nasopharyngeal, nasal, mid-turbinate and oropharyngeal swab specimens, nasopharyngeal wash/aspirate or nasal wash, and lower respiratory tract specimens.6
Personalized Respiratory Testing
The Panther Fusion assays provide the flexibility to run patient-specific targets, allowing for personalized patient testing and better cost control in your lab.
- The Panther Fusion® SARS-CoV-2/Flu A/B/RSV assay§ - Qualitative detection and differentiation of SARS-CoV-2, influenza A virus, influenza B virus and respiratory syncytial virus.1
- The Panther Fusion® Flu A/B/RSV assay - Qualitative detection and differentiation of influenza A virus, influenza B virus and respiratory syncytial virus.2
- The Panther Fusion® AdV/hMPV/RV assay - Qualitative detection and differentiation of adenovirus, human metapneumovirus and rhinovirus.3
- The Panther Fusion® Paraflu assay - Qualitative detection and differentiation of parainfluenza 1 virus, parainfluenza 2 virus, parainfluenza 3 virus and parainfluenza 4 virus.4
- The Panther Fusion® Bordetella assay† - Qualitative detection and differentiation of Bordetella pertussis and Bordetella parapertussis.5
- The Hologic® SARS-CoV-2 assays*‡ - Qualitative detection of SARS-CoV-2 virus.6-8
Flex Your Ability
With the Panther Fusion Respiratory assays, a single patient specimen can be tested for SARS-CoV-2 as well as other common respiratory viruses which present with overlapping symptoms, boosting efficiency and increasing clinical insight.
Additionally, when you leverage the power of Panther Fusion, your lab can:
- Run more efficiently with full automation from sample-to-result.
- Receive easy-to-interpret results.
- Personalize syndromic respiratory testing by processing multiple assays from a single specimen.
- Control costs by running only the required assays and reduce expense of unnecessary tests.
- Eliminate the need for reagent preparation with Panther Fusion’s ready-to-use format.
- Reduce waste with 60 day on-board reagent stability.
- Consolidate menu onto a single, fully automated platform with the ability to run both Aptima and Panther Fusion assays alongside each other at the same time.
Resources
Education