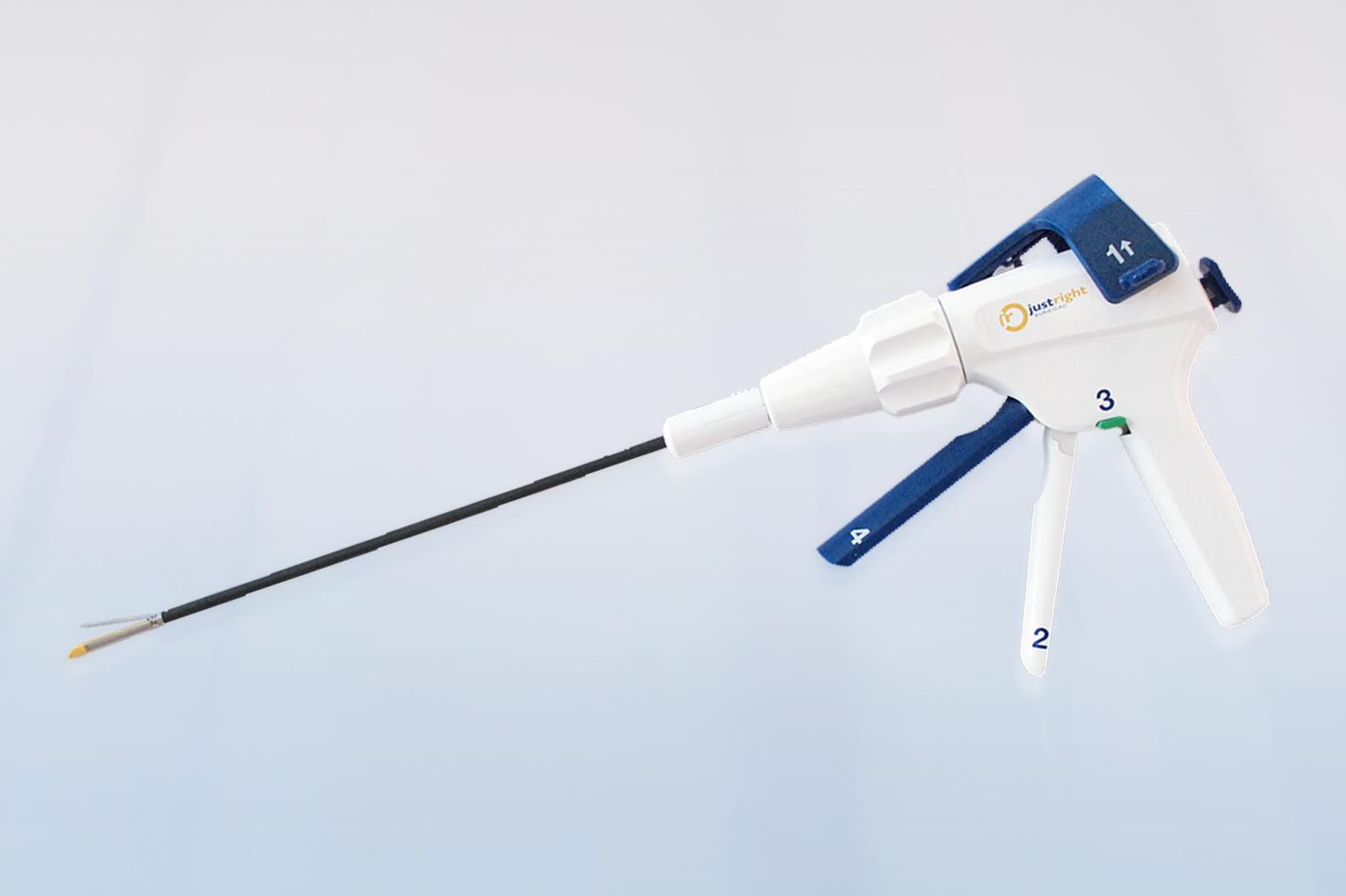
JustRight® 5mm Stapler
Elevating laparoscopic and open procedures with a one-of-a-kind 5mm stapling instrument.
Overview
Documents
Training
Revolutionizing Minimally Invasive Surgery
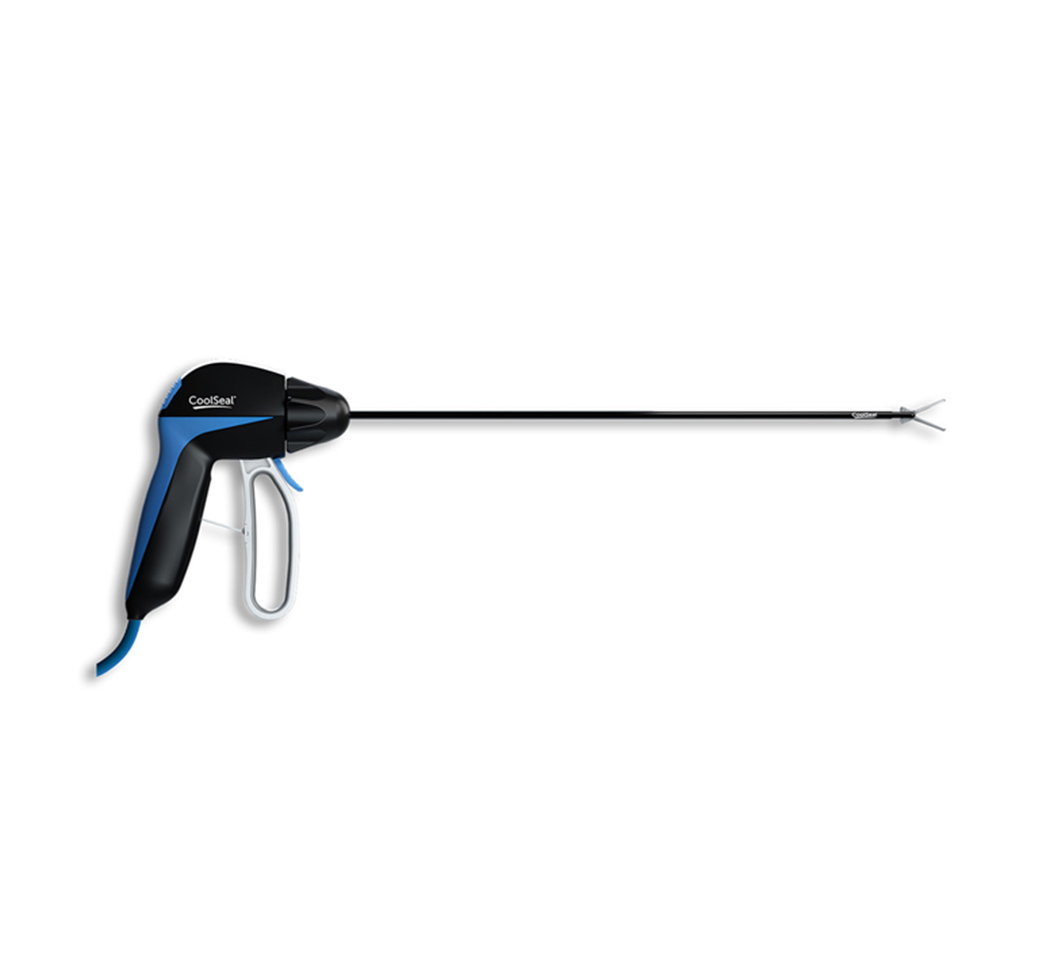
The JustRight stapler is the world's only 5mm stapling instrument with classic B-shaped staples, raising the bar in general and pediatric surgery. At less than half the size of other staplers, the optimized jaw length of the JustRight stapler allows for downsizing laparoscopic ports and eliminates the need to use oversized, overpowered instruments.1
Access Tighter Spaces
Tackle tight spaces with ease using 5mm shaft diameter - less than half the size of the standard 12mm and fitting down any conventional 5mm trocar.
Optimal port placement
Optimize your approach to the surgical site with flexibility in stapler placement with a 5mm shaft.
Enhanced Visualization
The jaw takes up less of the visible field in scope than the standard 12mm stapler, allowing a better view of the surgical field.

Patient Benefits of 5mm vs 12 mm Incision
Patients may experience:
- Minimal post-op pain2,3
- Faster recovery times2
- Minimal scarring3
- Minimal risk of post site herniation4,5
- Decreased potential for surgical site infection2,3
Small Device. Big Outcome.
Based on a study of pediatric thoracoscopic management, it was found that “the use of miniaturized instruments such as the CoolSeal 3 mm vessel sealing device and the JustRight 5 mm stapler allowed surgeons to perform a quicker and a safer procedure."1
Specialties
Pediatrics, Gynecology, Thoracic, Abdominal
Procedures
Lap Appendectomy, CPAM with Lobectomy, VATS, Colon resection, LADDS, Pull Through, Small bowel resection, Lap Cholecystectomy, Lap Nissen Fundoplication, Nephrectomy
Vizient Award
The CoolSeal® advanced energy portfolio and JustRight® 5mm stapler were awarded the Innovative Technology designation from Vizient, Inc., the nation’s largest provider-driven healthcare performance improvement company. Vizient awarded the contract after hospital experts who served on one of its provider-led councils recommended CoolSeal and JustRight.
These provider-led councils evaluate products submitted through Vizient’s Innovative Technology Program. They recommend contracts for technologies that have the potential to enhance clinical care, patient safety and healthcare worker safety, or to improve business operations of healthcare organizations.
View the Hologic press release.
Product Videos
1. JOURNAL OF LAPAROENDOSCOPIC & ADVANCED SURGICAL TECHNIQUES Volume 31, Number 3, 2021 2. Trocar Site Hernia. Hitoshi Tonouchi, MD, PhD; Yukinari Ohmori, MD; Minako Kobayashi, MD, PhD; Masato Kusunoki, MD, PhD, Arch Surg. 2004;139(11):1248-1256. 3. Incisions do not simply sum. Blinmin T. Surgical Endoscopy. 2010; 24(10):1746-52. 4. Trocar Site Hernia After Laparoscopic Cholecystectomy Uslu, H, Erkek, A, Cakmakm A, Kepenekci, I, Sozener, U, Kocaay, , Journal of Laparoendoscopic and Advanced Surgical Techniques, Volume 17, Number 5, 2007 5. Port-site incisional hernia: A case series of 54 patients. Lambertz, B.O. Stüben, B. Bock, R. Eickhoff, A. Kroh , C.D. Klink ,U.P. Neumann, C.J. Krones . Annals of Medicine and Surgery 14 (2017) 8e11
Related Products
Documents
Safety Data Sheets
Package Inserts

Overview
Package Inserts
Resources
Syndromic Respiratory Testing.
Patient-Specific Results.
The Panther Fusion Respiratory assays are the premier set of assays on the Panther Fusion® system. You can provide truly personalized syndromic respiratory testing with qualitative detection and differentiation of the most common respiratory viruses from a single patient sample. Each Panther Fusion Respiratory assay can be processed independently or simultaneously with other Panther Fusion® and Aptima® assays.
The Panther Fusion® SARS-CoV-2/Flu A/B/RSV, Panther Fusion® Flu A/B/RSV, Panther Fusion® Paraflu, Panther Fusion® AdV/hMPV/RV and Panther Fusion® Bordetella assays comprise the CE-IVD respiratory testing menu on the fully automated Panther Fusion system. Each is a multiplex, real-time PCR in vitro diagnostic test. These assays can be run on nasopharyngeal (NP) swab specimens obtained from individuals exhibiting signs and symptoms of a respiratory tract infection.1-5 Additionally, the Panther Fusion® SARS-CoV-2 assay has received Emergency Use Authorization for the detection of SARS-CoV-2 from nasopharyngeal, nasal, mid-turbinate and oropharyngeal swab specimens, nasopharyngeal wash/aspirate or nasal wash, and lower respiratory tract specimens.6
Personalized Respiratory Testing
The Panther Fusion assays provide the flexibility to run patient-specific targets, allowing for personalized patient testing and better cost control in your lab.
- The Panther Fusion® SARS-CoV-2/Flu A/B/RSV assay§ - Qualitative detection and differentiation of SARS-CoV-2, influenza A virus, influenza B virus and respiratory syncytial virus.1
- The Panther Fusion® Flu A/B/RSV assay - Qualitative detection and differentiation of influenza A virus, influenza B virus and respiratory syncytial virus.2
- The Panther Fusion® AdV/hMPV/RV assay - Qualitative detection and differentiation of adenovirus, human metapneumovirus and rhinovirus.3
- The Panther Fusion® Paraflu assay - Qualitative detection and differentiation of parainfluenza 1 virus, parainfluenza 2 virus, parainfluenza 3 virus and parainfluenza 4 virus.4
- The Panther Fusion® Bordetella assay† - Qualitative detection and differentiation of Bordetella pertussis and Bordetella parapertussis.5
- The Hologic® SARS-CoV-2 assays*‡ - Qualitative detection of SARS-CoV-2 virus.6-8
Flex Your Ability
With the Panther Fusion Respiratory assays, a single patient specimen can be tested for SARS-CoV-2 as well as other common respiratory viruses which present with overlapping symptoms, boosting efficiency and increasing clinical insight.
Additionally, when you leverage the power of Panther Fusion, your lab can:
- Run more efficiently with full automation from sample-to-result.
- Receive easy-to-interpret results.
- Personalize syndromic respiratory testing by processing multiple assays from a single specimen.
- Control costs by running only the required assays and reduce expense of unnecessary tests.
- Eliminate the need for reagent preparation with Panther Fusion’s ready-to-use format.
- Reduce waste with 60 day on-board reagent stability.
- Consolidate menu onto a single, fully automated platform with the ability to run both Aptima and Panther Fusion assays alongside each other at the same time.
Resources
Education