Watch our sizzle from ID Lab Con!

Technology session
Tuesday March 14th,
7:45–8:15 am
Karen Harrington, PhD HCLD (ABB),
Head of Scientific Affairs
Hologic, Inc.
Evolving HCV epidemic:
Role of HCV testing in closing the gap and meeting 2030 CDC elimination goals
Hepatitis C virus (HCV) infection remains a substantial health problem as a leading cause of chronic liver disease worldwide. This session will evaluate HCV screening and testing rates, discuss challenges with optimizing diagnosis of current HCV infection and ensuring linkage to treatment for individuals with HCV.
Get a deeper dive into the data with poster presentations
1. Optimization and Verification of an Automated Analyte-specific Reagent Assay for Mycoplasmoides genitalium Macrolide Resistance Detection in Primary Clinical Specimens Erik Munson1, Amanda Zapp1, Stephen Lavey2, Hunter Russell1, Kimber L. Munson3; 1. Marquette University, Milwaukee, Wisconsin; 2. Carleton College, Northfield, Minnesota; 3. Ascension Wisconsin, Milwaukee, Wisconsin
2. Evaluation of Updated Aptima Combo 2 Assay in a High-prevalence United States Sexually-transmitted Infection Community Manjeet Khubbar1, Zorangel Netterfield-Amezquita1, Carissa Ahrenhoerster1, Katherine Akinyemi1, Amy Bauer1, Erik Munson2; 1. Milwaukee Health Department Laboratory, Milwaukee, Wisconsin; 2. Marquette University, Milwaukee, Wisconsin
3. Evaluation of Analyte Specific Reagents for Molecular Detection of Macrolide Resistance in Mycoplasma genitalium Olusegun O. Soge1-3,5, Erika Wakatake1, Gwen Wood2,5, Ejovwoke Ememu7, Godfred Masinde7, Matthew R. Golden1,2,4-6, Lisa Manhart1,4,5; 1. Department of Global Health, University of Washington, Seattle, WA, USA; 2. Department of Medicine, University of Washington, Seattle, WA, USA; 3. Department of Laboratory Medicine and Pathology, University of Washington, Seattle, WA, USA; 4. Department of Epidemiology, University of Washington, Seattle, WA, USA; 5. Center for AIDS & STD, University of Washington, Seattle, WA; 6. Public Health – Seattle & King County, HIV/STD Program, Seattle, WA, USA; 7. San Francisco Public Health Laboratory, San Francisco, CA
Old Pathogens Meet New Testing Practices
Updates to STI and HIV testing routines


This year in Atlanta, we would like to introduce you to our fully-automated Panther® system, the foundation of Panther® Scalable Solutions.
We would love to discuss in person how Panther® Scalable Solutions can help streamline your lab’s workflow and customize molecular diagnostic testing. With the Panther® system you can choose from a broad assay menu and a suite of instrument add-ons, providing an economical and scalable path to your laboratory’s growth.
Discover how Panther® Scalable Solutions can meet your needs in molecular diagnostic testing today and also prepare your lab for an unpredictable future by reducing complexities and decreasing turnaround times.
19
Assays available
12
More in development
1
Scalable Platform


Unlock your lab's potential and learn more about Panther Fusion® Open Access™
Laboratory Developed Tests (LDTs) are an essential diagnostic tool for Public Health Laboratories.
Patients with certain medical conditions depend on these in-house developed tests when other options are not available. Save time and increase efficiency by consolidating your LDTs and IVD assays on the fully automated, sample-to-result, Panther Fusion system using the Open Access™ functionality.
What’s in the way of Africawide molecular diagnostic roll out?
How are molecular diagnostics expanding access to diagnostic testing for viral diseases in low resource settings? Devex investigates.

Public health labs are at the forefront of the battle to diagnose HIV & Hepatitis
Together we can improve care where it is needed most
No matter the size of your lab, you can support your clinicians with reliable, accurate results with the Aptima Virology assays.
The Aptima® HCV Quant Dx assay is standardized against the 2nd WHO International Standard for Hepatitis C Virus (NIBSC Code 96/798). Targeting, amplification, and detection are all conducted on the Panther system.
The Aptima HIV-1 Quant Dx assay is a dual-claim assay that can be used to confirm HIV-1 infection and measure viral load for optimal patient management.
The Hologic Global Access Initiative is a partnership with the Clinton Health Access Initiative, Inc. (CHAI) and MedAccess (backed by the UK government) to mitigate the burden of viral diseases in areas with high prevalence by providing greater access to testing using the Panther system.
Visit Hologic Booth 215/217 to discuss how Hologic is involved!

Visit Hologic Booth 215/217 and learn more about personalized respiratory testing
The Panther Fusion respiratory assays provide the flexibility to run patient-specific targets, allowing for personalized patient testing and better cost control in your lab.
- The Panther Fusion Flu A/B/RSV assay - Qualitative detection and differentiation of influenza A virus, influenza B virus and respiratory syncytial virus.1
- The Panther Fusion AdV/hMPV/RV assay - Qualitative detection and differentiation of adenovirus, human metapneumovirus and rhinovirus.2
- The Panther Fusion Paraflu assay - Qualitative detection and differentiation of parainfluenza virus 1-4.3
- The Hologic® SARS-CoV-2 assays* - Qualitative detection of SARS-CoV-2 virus.4
Comparative analysis of four sample-to-result influenza A/B/RSV nucleic acid amplification assays using adult respiratory specimens.


Laboratory Professionals Play an Essential Role in the Fight Against STIs
Mycoplasma genitalium (M. gen) is a highly prevalent sexually transmitted infection (STI). The CDC recommends M. gen testing for patients with recurring cervicitis and urethritis.5
The test you choose matters
The Aptima® Mycoplasma genitalium assay is a nucleic acid amplification test (NAAT) that enables efficient and reliable testing by detecting rRNA.
The sensitivity of RNA-based tests is needed for accurate diagnosis of M. gen. An RNA-based test accurately identified the 40% of patients missed by a DNA-based test.6
Visit us at Hologic Booth 215/217 to expand your testing menu.
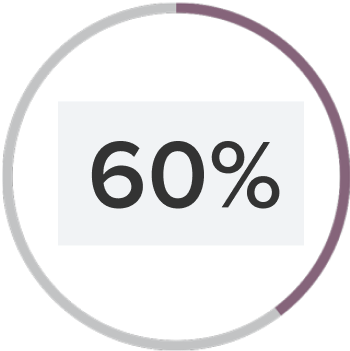
DNA-based tests can miss 40% of infections compared to rRNA-based tests.6

rRNA-based Aptima Mycoplasma genitalium assay provides up to 100% sensitivity.6
Assay Menu
Women's Health
Candida vaginosis/Trichomonas vaginalis
Zika Virus*
GBS†
M. gen macloide resistance†
Chlamydia trachomatis†
Neisseria gonorrhoeae†
Infectious Disease
SARS-CoV-2Flu/A/B/RSV†
GI Bacterial†
GI Extended Bacterial†
GI Viral†
GI Parasite†
EBV†
BKV†
C. Difficule†