Overview
Documents
Training
Transform Respiratory Specimen Processing
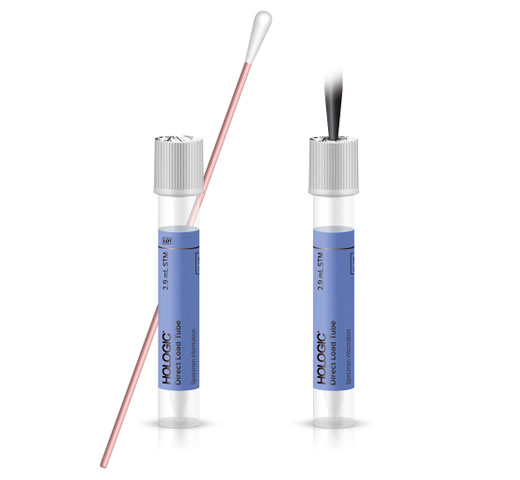
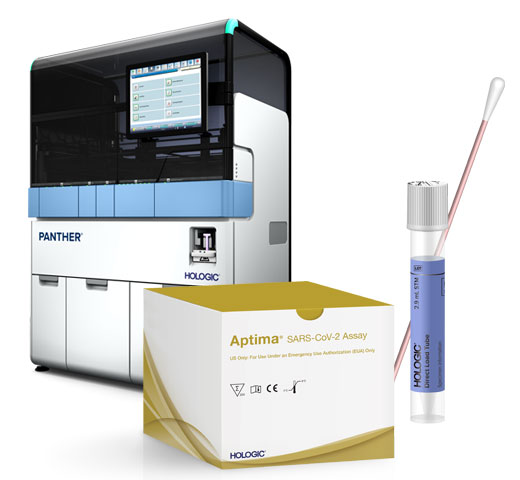
Introducing Hologic’s new RespDirect™ Collection Kit. Samples arrive in the lab in an enhanced Direct Load Tube (eDLT) which enables laboratories to directly load samples for processing on the Panther Fusion system without any uncapping or specimen transfer steps.
This primary collection device is validated for use with:
Panther Fusion SARS-CoV-2/Flu A/B/RSV assay (NP samples)
Aptima SARS-CoV-2 assay* (EUA) (Nasal and NP samples)


Deliver Results Sooner
Expedite turnaround time using random and continuous access to load specimens for respiratory testing alongside samples for other Aptima and Panther Fusion assays, without batching. Prepare for the unpredictable testing surges associated with respiratory season with automation capable of processing over 1,000 samples per day.