Overview
Documents
Training
Empower Your Genius With Ours
The Genius Digital Diagnostics System is the first and only FDA cleared digital cytology system – combining a new artificial intelligence (AI) with advanced volumetric imaging technology to help cytologists and pathologists identify pre-cancerous lesions and cervical cancer cells.
Clinical Benefits
Screening for cervical cancer using Genius Digital Diagnostics provides more sensitive disease detection than manual and image-guided review.1
Streamlined Efficiency
Digital imaging and AI-guided review significantly streamlines laboratory workflows, supporting staff and reducing costs.
Innovation
Genius Digital Diagnostics offers cytologists and pathologists innovative technology that supports the future of cytology and co-testing as the most sensitive cervical cancer screening strategy.2-5
Make a Greater Impact on Cervical Cancer
7.5%
increase in HSIL+ sensitivity vs. manual review1
3.6%
increase in HSIL+ sensitivity vs. ThinPrep® Imaging System review1
Transform screening with artificial intelligence, advanced imaging, and digital efficiency.
Video Resources
1. Genius Digital Diagnostics System with Genius Cervical AI. US Instructions for Use AW-23890-001. Hologic, Inc.; 2024. 2. Austin RM, et al. Enhanced detection of cervical cancer and precancer through use of imaged liquid-based cytology in routine cytology and HPV co-testing. Am J Clin Pathol.2018;150(5):385-392. doi: 10.1093/ajcp/aqy114 (Study included the ThinPrep® Pap test, ThinPrep imaging, digene HPV, Cervista HPV and Aptima HPV). 3. Blatt AJ, et al. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123(5):282-288. doi:10.1002/ cncy.21544 (Study included ThinPrep, SurePath and Hybrid Capture 2 assay). 4. Kaufman H, et al. Contributions of Liquid-Based (Papanicolaou) Cytology and Human Papillomavirus Testing in Cotesting for Detection of Cervical Cancer and Precancer in the United States. Am J Clin Pathol. 2020:XX:0-0 DOI: 10.1093/AJCP/AQAA074 (Study included ThinPrep Pap test, ThinPrep imaging, SurePath Pap test, SurePath imaging, Aptima HPV assay and Hybrid Capture 2 HPV assay). 5. Zhou H, et al. Clinical performance of the Food and Drug Administration-Approved high-risk HPV test for the detection of high-grade cervicovaginal lesions. Cancer Cytopathol. 2016 May;124(5):317-23. doi: 10.1002/cncy.21687. (Study included Cobas HPV, SurePath and ThinPrep).
Safety Data Sheets
Package Inserts
Related Products
Hologic introduces the Genius Digital Diagnostics System*
Genius Digital Diagnostics is the first CE-marked digital cytology platform to combine a new artificial intelligence (AI) algorithm with advanced volumetric imaging technology to help cytotechnologists and pathologists identify pre-cancerous lesions and cancer cells in women.
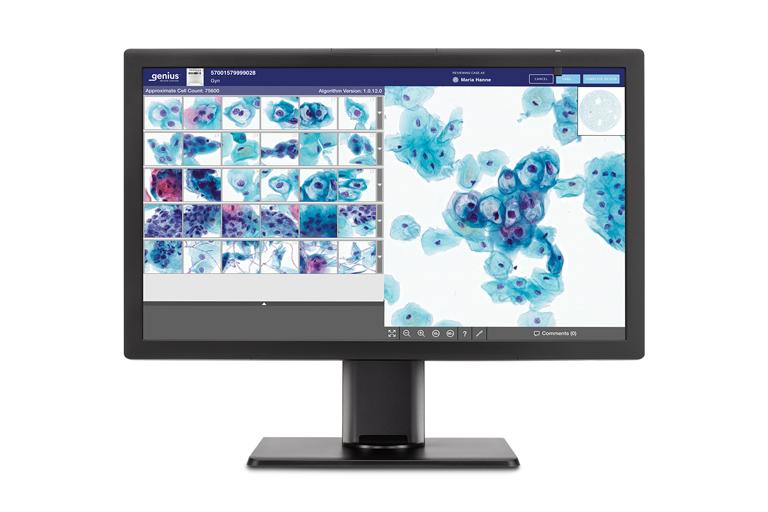
The system can rapidly analyze all cells on a ThinPrep® Pap test digital image, narrowing tens of thousands of cells down to an AI-generated gallery of the most diagnostically relevant images. This will help provide healthcare providers with the critical information they need to guide earlier detection and better treatment decisions for the patients they serve.
* Genius Digital Diagnostics is CE-marked for diagnostic use in Europe. May not be available in all markets. Contact your local Hologic representative for availability in your country.
The only comprehensive cervical cancer screening portfolio from sample collection to digital diagnosis.
The Genius Digital Diagnostics System’s cutting-edge technology includes:
Image Clarity
An advanced digital imager featuring volumetric imaging technology that produces exceptional image clarity.
Secure Image Management
A secure image management server for storing images.
New AI Algorithm
A new, deep learning-based artificial intelligence algorithm that is designed to detect pre-cancerous lesions and cervical cancer cells.
Review Station
A high-resolution review station for local or remote case review.
Explore the system
Genius Digital Diagnostics enables seamless and dynamic collaboration across laboratories within a network, connecting pathologists with remote review so each patient can benefit from the collective knowledge of geographically dispersed experts when needed. Digital case review promises to enhance the experience for lab partners by improving workflow and accelerating review time.