Overview
Documents
Training
Unlock Your Lab's Potential
Laboratory Developed Tests (LDTs) are an essential part of diagnostics. Patients with certain medical conditions depend on these in-house developed tests when other options are not available.
Save time and increase efficiency by consolidating your LDTs and IVD assays on the fully automated, sample-to-result, Panther Fusion system using the Open Access® functionality.
Customize Your LDTs
Gain the following incremental benefits without increasing the footprint of your Panther Fusion system.
- Create unique qualitative or quantitative LDT protocols using the myAccess software.
- Multiplex up to 5 targets in a single PCR reaction.
- Increase efficiency and minimize waste with individual, unitized ready-to-use reagent cartridges and 60 days of on-board stability.
Streamline Your LDT Workflow
Follow these steps to design LDT protocols using the myAccess software.
- Create Panther Fusion® Open Access® Protocol using the myAccess software.
- Load reagents
- Load patient specimens
- Review results with option to automatically release to LIS.
Configurable Testing Based on Your Needs
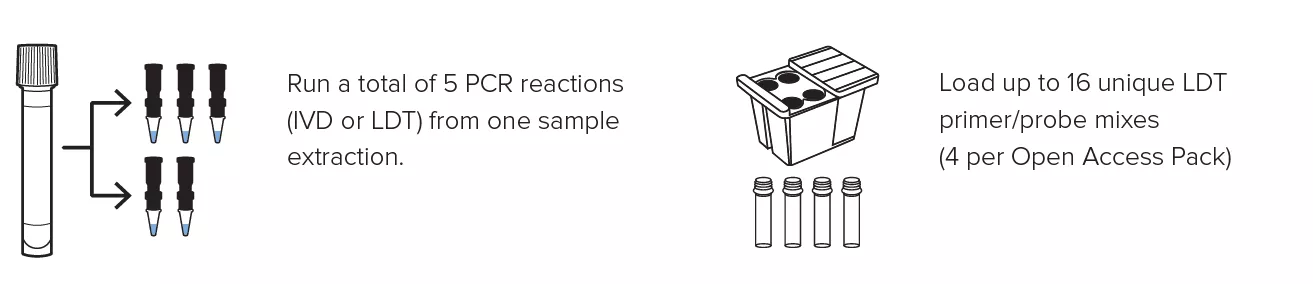
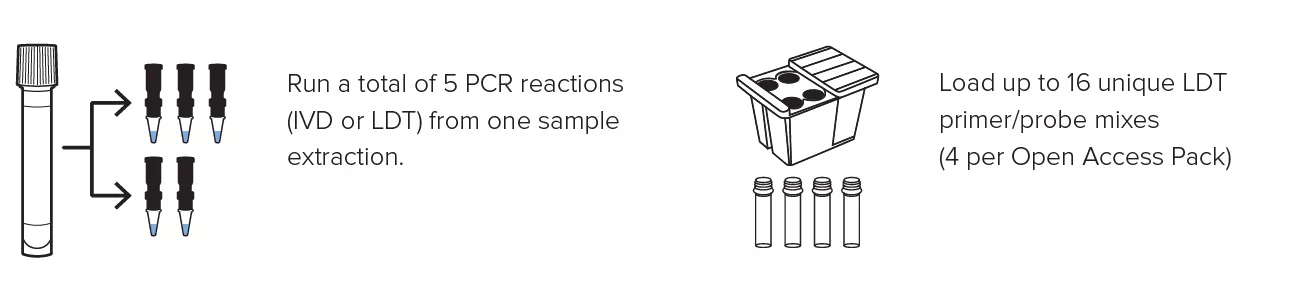
Create personalized panels with the ability to run up to 16 LDTs at once and obtain up to 5 different PCR reactions from a single specimen extraction.
Panther Fusion Open Access is a non-IVD function that is not required to have FDA clearance or approval.
1. Panther Fusion System Open Access Application Sheet. AW-23623-001. Hologic, Inc.; 2017-2022.
Safety Data Sheets
Package Inserts
Related Products

Overview
Package Inserts
Unlock Your Lab's Potential
Laboratory Developed Tests (LDTs) are an essential part of diagnostics. Patients with certain medical conditions depend on these in-house developed tests when other options are not available.
Save time and increase efficiency by consolidating your LDTs and IVD assays on the fully automated, sample-to-result, Panther Fusion® system using the Open Access™ functionality.
Customize Your LDTs
Gain the following incremental benefits without increasing the footprint of your Panther Fusion system.
- Create unique qualitative or quantitative LDT protocols using the myAccess software.
- Multiplex up to 5 targets in a single PCR reaction.
- Increase efficiency and minimize waste with individual, unitized ready-to-use reagent cartridges and 60 days of on-board stability.
Streamline Your LDT Workflow
Follow these steps to design LDT protocols using the myAccess software.
-
Create Panther Fusion® Open Access Protocol™ using the myAccess software.
-
Load reagents
-
Load patient specimens
-
Review results with option to automatically release to LIS.
Configurable Testing Based on Your Needs
Create personalized panels with the ability to run up to 16 LDTs at once and obtain up to 5 different PCR reactions from a single specimen extraction