Instructions for Using the Hologic® RespDirect™
Collection Kit for Patient Collected Specimens
Instructions for Use
NASAL SPECIMEN COLLECTION
GENERAL INSTRUCTIONS FOR NASAL SWAB SPECIMEN COLLECTION AND HANDLING
Carefully follow procedures for reliable results. If you have any questions about any procedure, ask your health-care provider.
1. Wash your hands before starting. If soap and water are not available, use hand sanitizer.
2. Open kit package. Remove the swab and the tube. Set the tube aside before beginning instructions below to collect the specimen or specimens requested by your health-care provider.
3. Use the provided swab only. Failure to use the provided swab may invalidate the test results.
4. Do not use if the swab, tube, or packaging is visibly damaged (e.g., if the swab tip or shaft is bent or broken).
5. Do not bend or shape the swab before collection. Do not use excessive force, pressure or bending when collecting the specimen as this may result in accidental breakage of the swab.
6. Do not apply the contents in the transport tube directly to skin or mucous membranes or take internally.
7. If the contents of the transport tube are spilled at any time during the collection procedure, collect a new specimen using a new Hologic RespDirect Collection Kit. Failure to use a new kit may invalidate the test results.
8. Do not use the kit after expiration date to collect specimens.
NASAL SWAB SPECIMEN COLLECTION AND HANDLING
Note to Patient: If you have any questions about this procedure, please ask your healthcare provider.
Instructions for nasal swab specimen collection:

1. Partially peel open the swab package. Remove the swab. Do not touch the soft tip or lay the swab down. If the soft tip is touched, laid down, or dropped, discard and get a new Hologic RespDirect Collection Kit.
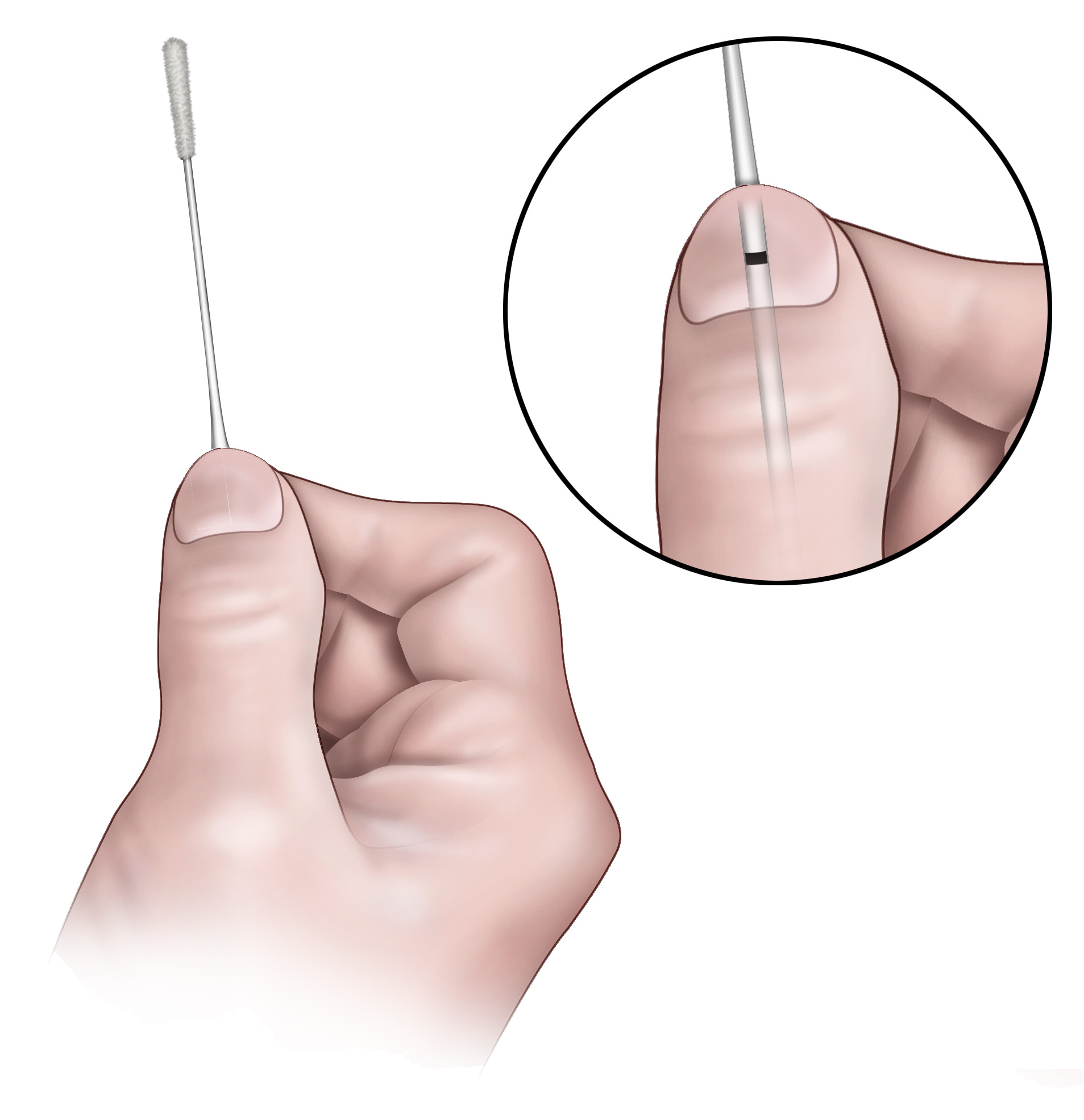
2. Hold the swab, placing your thumb and forefinger at the score line.
Note: Actual product may appear slightly different from the one shown in this section.
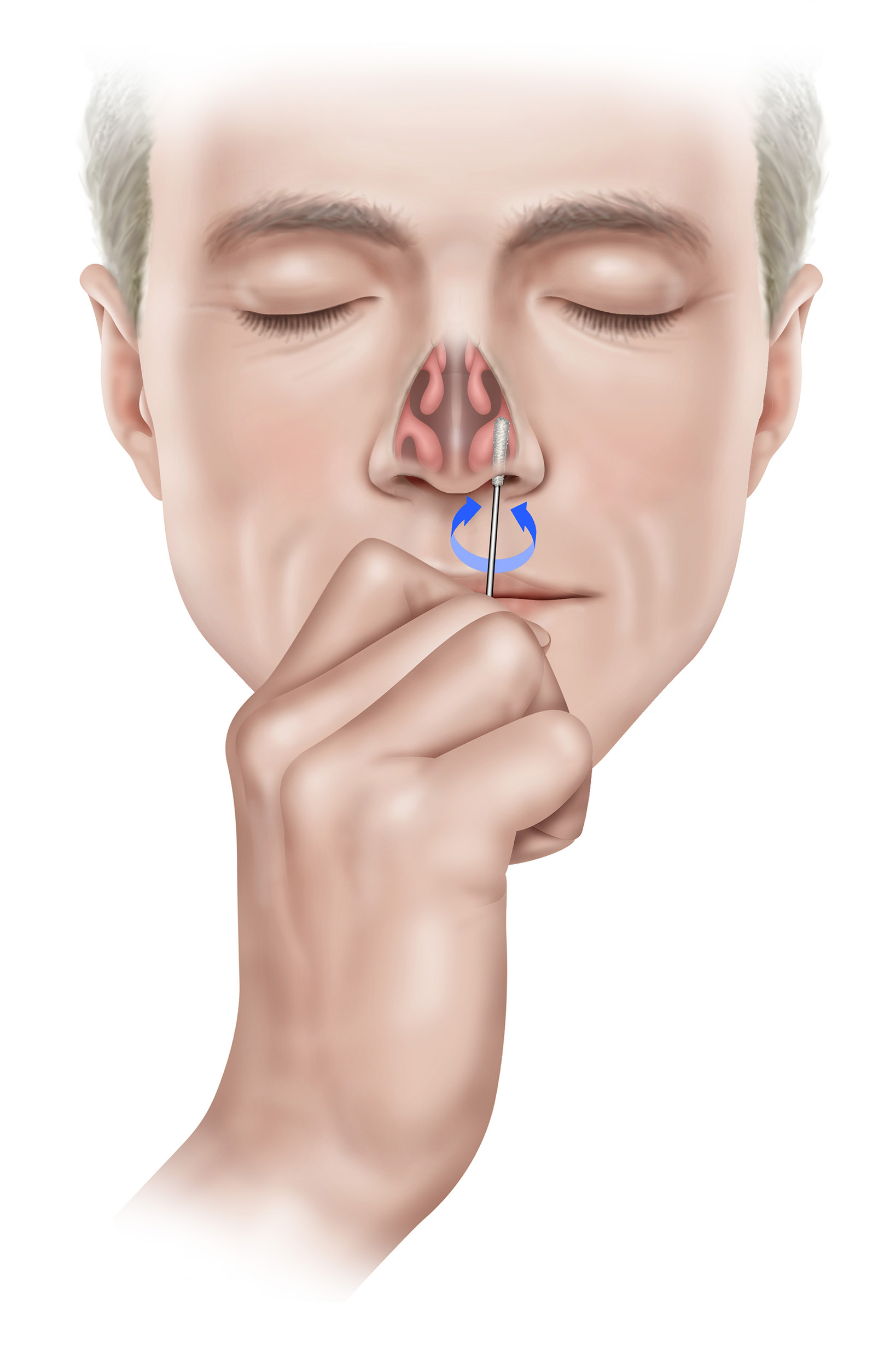
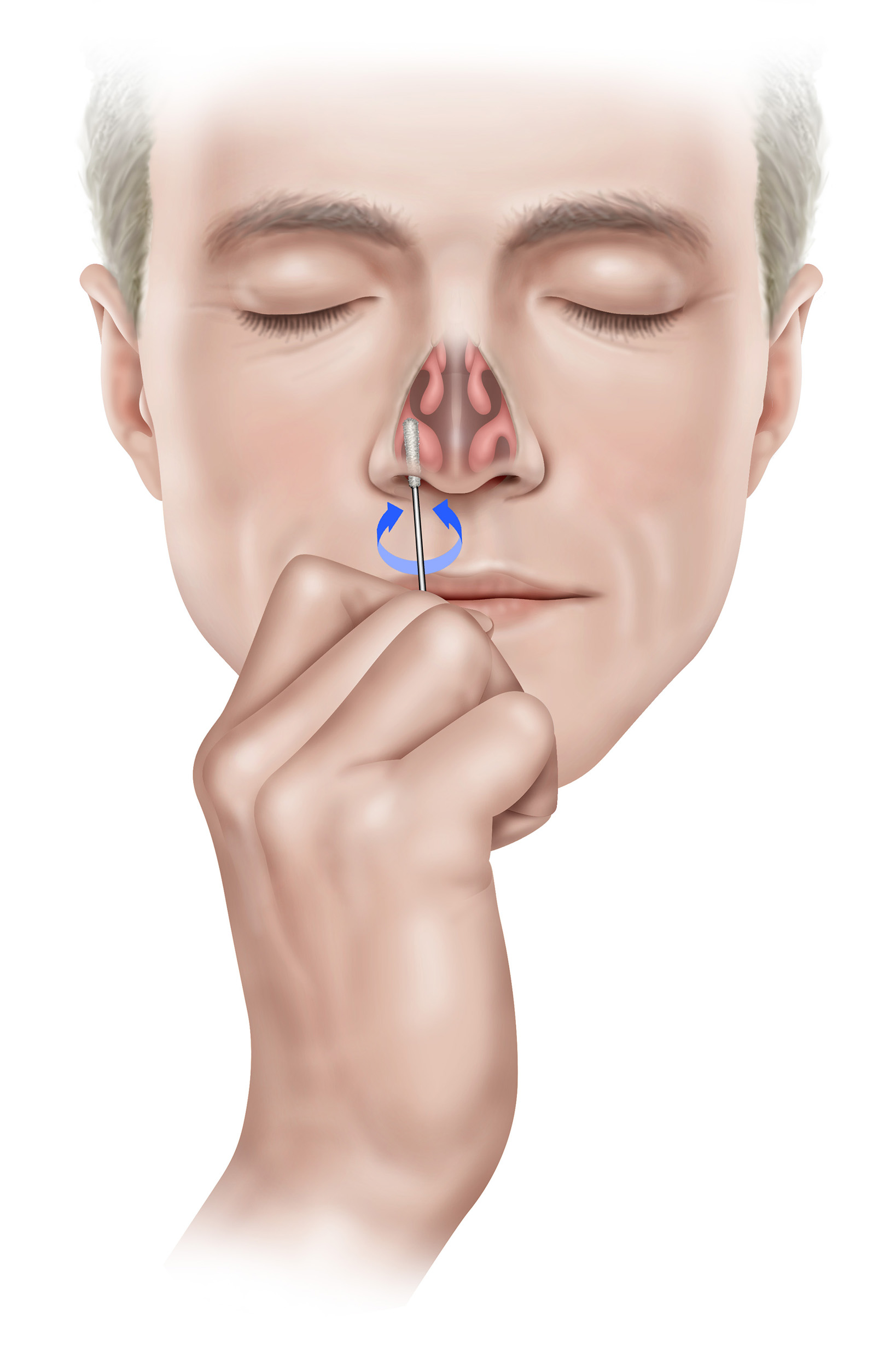
3. Carefully insert the swab into the first nostril until the swab tip is no longer visible, i.e. ½ to ¾ of an inch or 1.25 to 2 cm into the nostril. Rotate the swab with moderate pressure against as much of the wall of the nostril as possible in a large circular path inside the nose at least 4 times (approximately 10-15 seconds) against the nasal wall and remove from nostril. Repeat collection in the other nostril using the same swab. 1
Note: Simply twirling the swab against one part of the inside of the nose or leaving the swab in the nose for 10-15 seconds, is not proper technique and may result in an insufficient sample.
Note: You must collect the sample from both nostrils.
4. While holding the swab in the same hand, unscrew the cap from the tube. Do not spill the contents of the tube. If the contents of the tube are spilled, collect a new specimen using a new Hologic RespDirect Collection Kit.
Warning: If you come into contact with liquid from the tube, wash your hands with soap and water for 30 seconds.
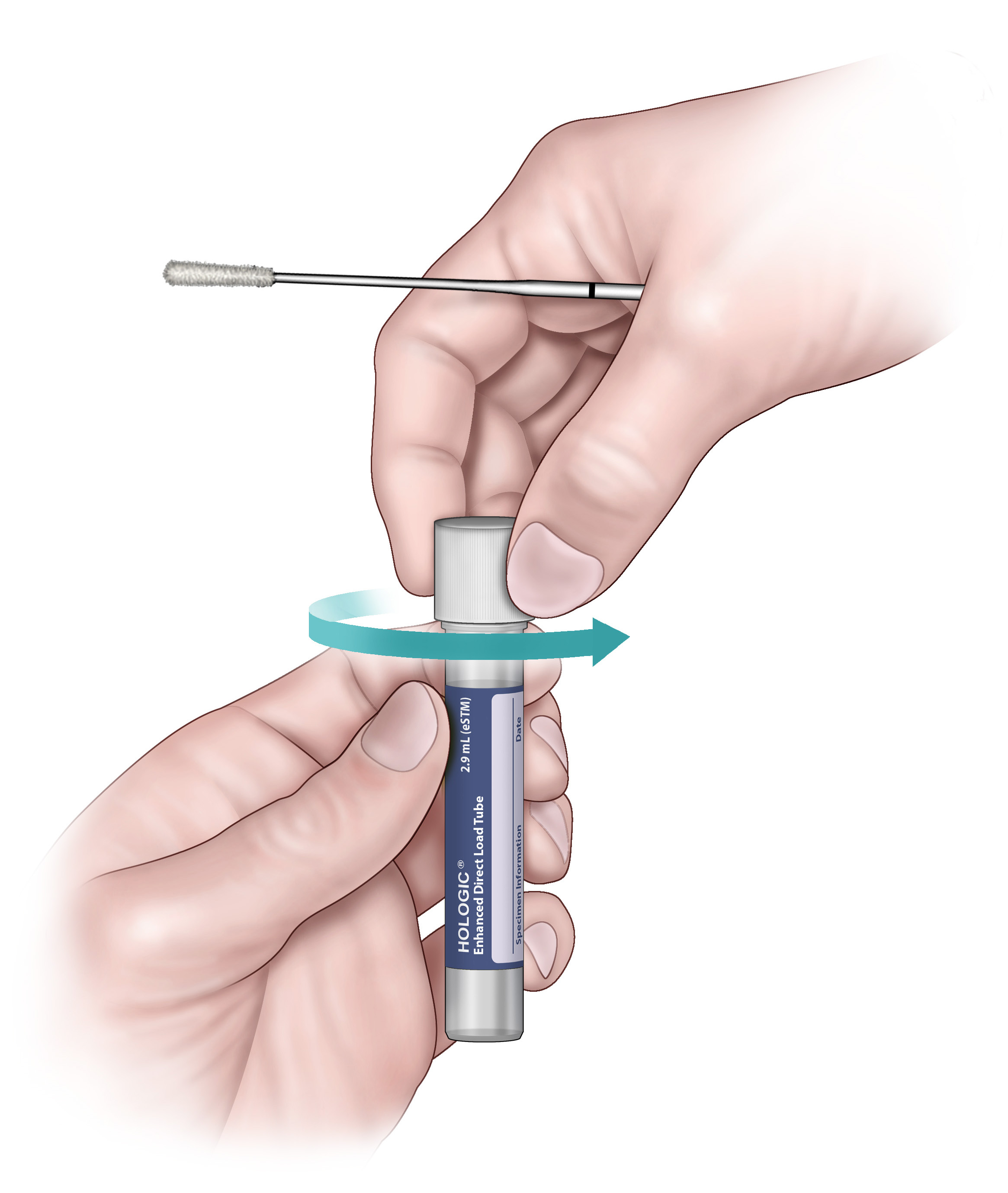
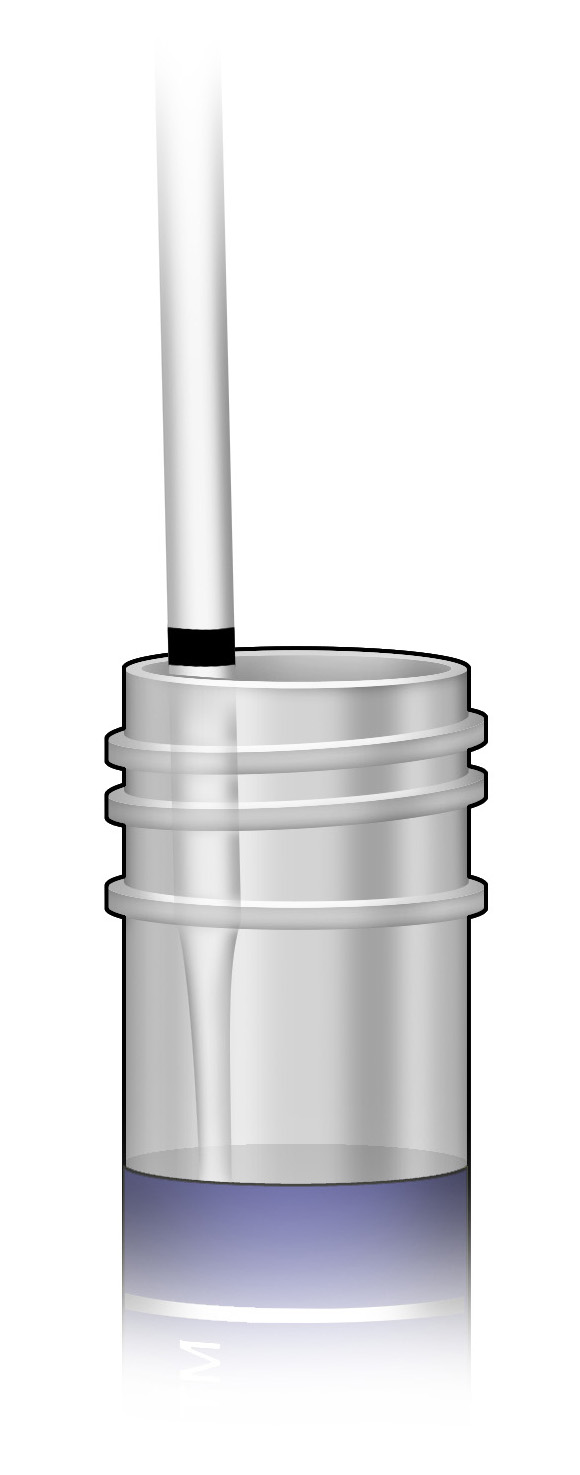
5. Immediately place the swab into the transport tube so that the score line is at the top of the tube.
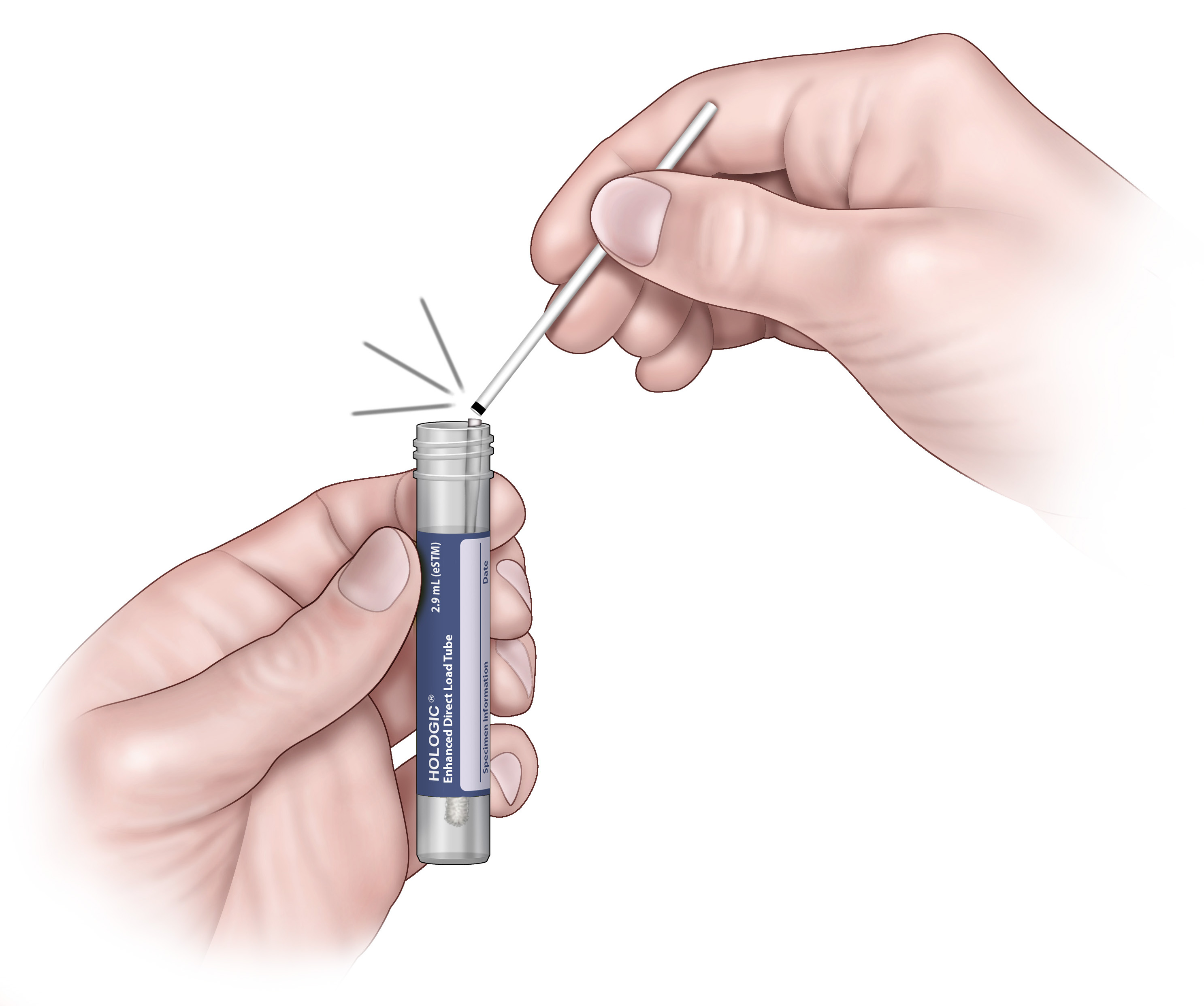
6. Carefully break the swab shaft at the score line against the side of the tube.
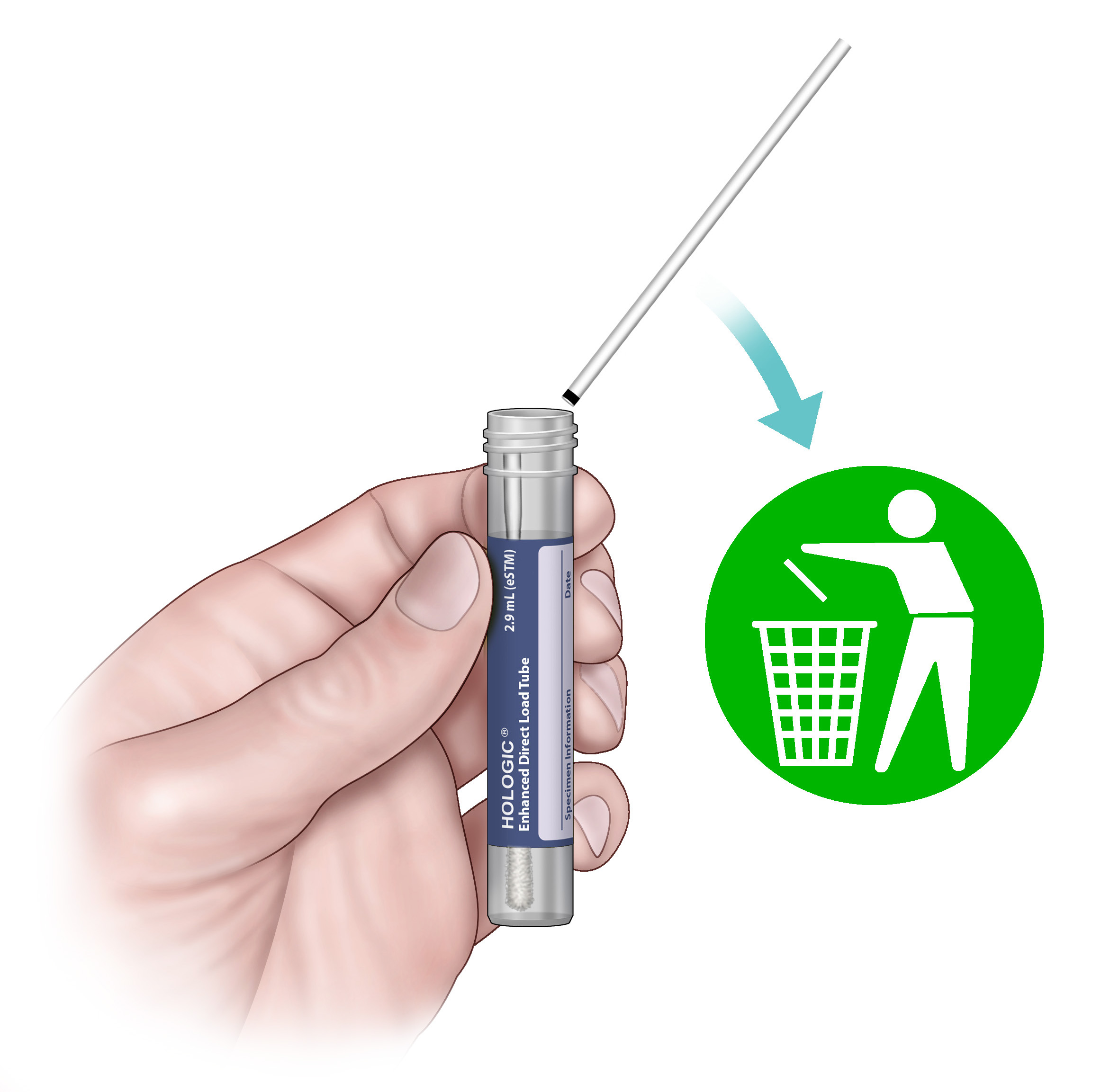
7. Discard the top portion of the swab shaft.
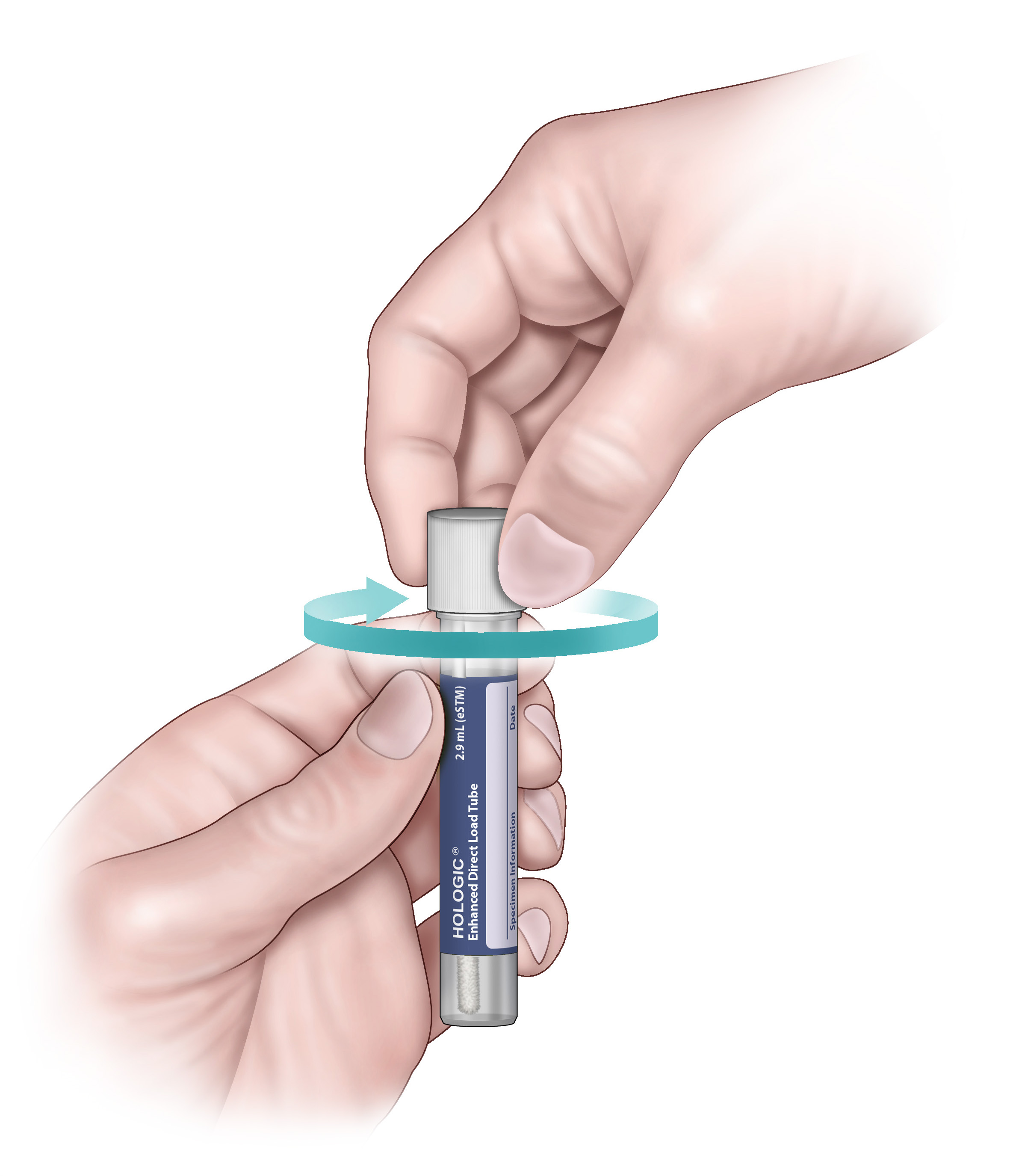
8. Tightly screw the cap into the tube.
Note: The tube label includes a field to record the specimen source.
1https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
Contact Information and Revision History

For country-specific Technical Support and Customer Service email address and telephone number, visit www.hologic.com/support.
Serious incidents occurring in relation to the device in the European Union should be reported to the manufacturer and the competent authority of the Member State in which the user and/or the patient is established.
Hologic and associated logos are trademarks and/or registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and/or other countries.
All other trademarks that may appear in this package insert are the property of their respective owners.
This product may be covered by one or more U.S. patents identified at www.hologic.com/patents.
©2023 Hologic, Inc. All rights reserved.
AW-29023-001 Rev. 001
2023-05


