Be prepared to face and confidently manage the uncertainty
To help you provide your patients with a timely diagnosis this respiratory season, Hologic’s targeted respiratory panels for the Panther Fusion® System ensure you can confidently meet the seasonal demands and prepare your lab for whatever it may bring.
Manage the increased demand throughout the upcoming respiratory season using Hologic’s flexible and reliable solution for combined respiratory and COVID testing.
Build your resilience to cope, streamline workflow and drive productivity with a respiratory testing system that provides speed and efficiency when it matters most.
Test for the most common respiratory viruses with ease
As the respiratory season holds many unknowns, spikes in respiratory infections are difficult to predict. By consolidating your lab's testing capabilities with the Panther Fusion® System, you can confidently manage the uncertainty of the seasonal surges.1
The Panther Fusion® SARS-CoV-2/Flu A/B/RSV assay enables simultaneous testing for the detection and differentiation of the most common respiratory viruses to help you deliver the essential testing that the season demands.1
In addition, further bolster your testing capabilities with the broader respiratory portfolio from Hologic, including Panther Fusion® AdV/hMPV/RV for detection and differentiation of Adenovirus, Human Metapneumovirus and Rhinovirus,2 Panther Fusion® Paraflu for detection and differentiation of Parainfluenza viruses 1,2,3,43 and Panther Fusion® Bordetella for detection and differentiation of B. pertussis and B. parapertussis.4
Our solutions can help your lab to alleviate the pressure of the respiratory season as assays can be processed independently or concurrently with other Panther Fusion® and Aptima® assays, including Aptima® SARS-CoV-2 and Aptima® SARS-CoV-2/Flu, which helps to boost efficiency and productivity preparing your lab for multiple scenarios and delivering a reliable service to your patients.2

Discover the Panther Fusion® SARS-CoV-2/FluA/B/RSV assay

- A comprehensive and fully-automated solution that enables optimal patient management and infection control1
- Supports efficient lab workflow with full automation from sample to result1
- Sample throughput of up to 500 tests in eight hours increases test volume per shift without increasing staff1
- Run multiple assays from a single patient specimen to detect the most common respiratory pathogens1
- Control costs by running only the required assays and reduce the expense of unnecessary tests1
Coming Soon
Simplified workflow with RespDirect Collection Kit* & Panther Fusion® SARS-CoV-2/FluA/B/RSV
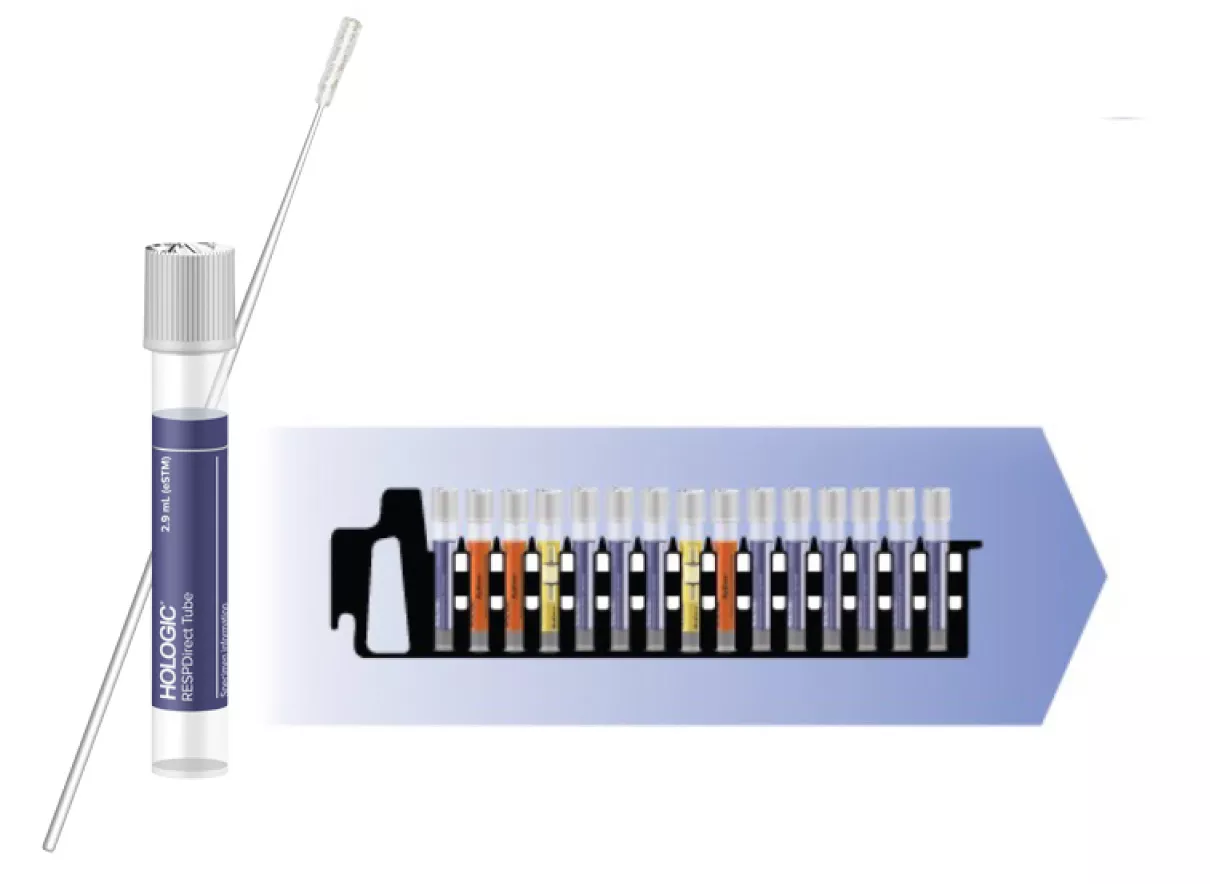
Directly load samples collected with the RespDirect Collection Kit alongside specimen types for other assays with true random and continuous access.

- No uncapping
- No sample transfer
- Inactivate sample

Join us for our webinar series!
Listen to experts from across Europe as they discuss a range of topics, including:
- Lessons learnt with high-throughput testing
- Real case studies and assay performance data
- Past respiratory seasons
- Predictions for the role of respiratory diseases as we move into winter, and beyond
Learn how Hologic can help support your lab's response to unknown respiratory threats by registering your interest today.
Read the trends in multiplexing by our Medical Director, Dr. Sakari Jokiranta
Trends in multiplexing: Preparing for the winter respiratory epidemic
Dr. Sakari Jokiranta. Medical Director, Hologic Diagnostic
Contact Us
Please provide your details below for more information on how Hologic can support the increased demand for your lab's testing capabilities this respiratory season.
References: 1. Panther Fusion SARS-CoV-2/Flu A/B/RSV Assay [package insert]. AW-25328-001, Rev. 01. San Diego, CA: Hologic, Inc 2. Panther Fusion AdV/hMPV/RV; AW-23710-001 Rev. 001 3. Panther Fusion Paraflu; AW-23708-001 Rev. 001 4. Panther Fusion Bordetella assay. Package insert AW-18637. Hologic, Inc.; 2018.
![]() Hologic BV, DA Vincilaan 5, 1930 Zaventem, Belgium. NB Number wherever applicable. EC REP information wherever applicable.
Hologic BV, DA Vincilaan 5, 1930 Zaventem, Belgium. NB Number wherever applicable. EC REP information wherever applicable.
WEB-01768-EUR-EN Rev 001 © 2023 Hologic, Inc. All rights reserved. Hologic and associated logos are trademarks and/or registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and/or other countries. All other trademarks are the property of their respective owners. This information is intended for medical professionals and is not intended as a product solicitation or promotion where such activities are prohibited. Because Hologic materials are distributed through websites, podcasts, and tradeshows, it is not always possible to control where such materials appear. For specific information on what products are available for sale in a particular country, please contact your Hologic representative or write to euinfo@hologic.com.
The content on this page is directed to audiences in Europe only.
*RespDirect not CE marked. Not available for sale.

