Overview
Documents
Publications
Training
Leading the charge in cervical disease prevention with the ThinPrep® system and Aptima® HPV assays
Hologic has remained an unwavering advocate for women’s health for more than two decades. Our goals as a company are intrinsically tied to changes in best-practices for women’s health, applying the latest findings in diagnostic medicine to the development of new products and technologies in response to the emergence of new discoveries in medicine.
Cervical disease screening is an essential component of our efforts in women’s health. Hologic is the leader in Pap and human papillomavirus (HPV) testing. The ThinPrep Pap test helps healthcare providers and laboratory professionals detect the presence of abnormal cervical cells, and the Aptima HPV assays identify high-risk HPV mRNA that is indicative of the HPV infections most likely to lead to cervical disease.1-3
Know the Facts
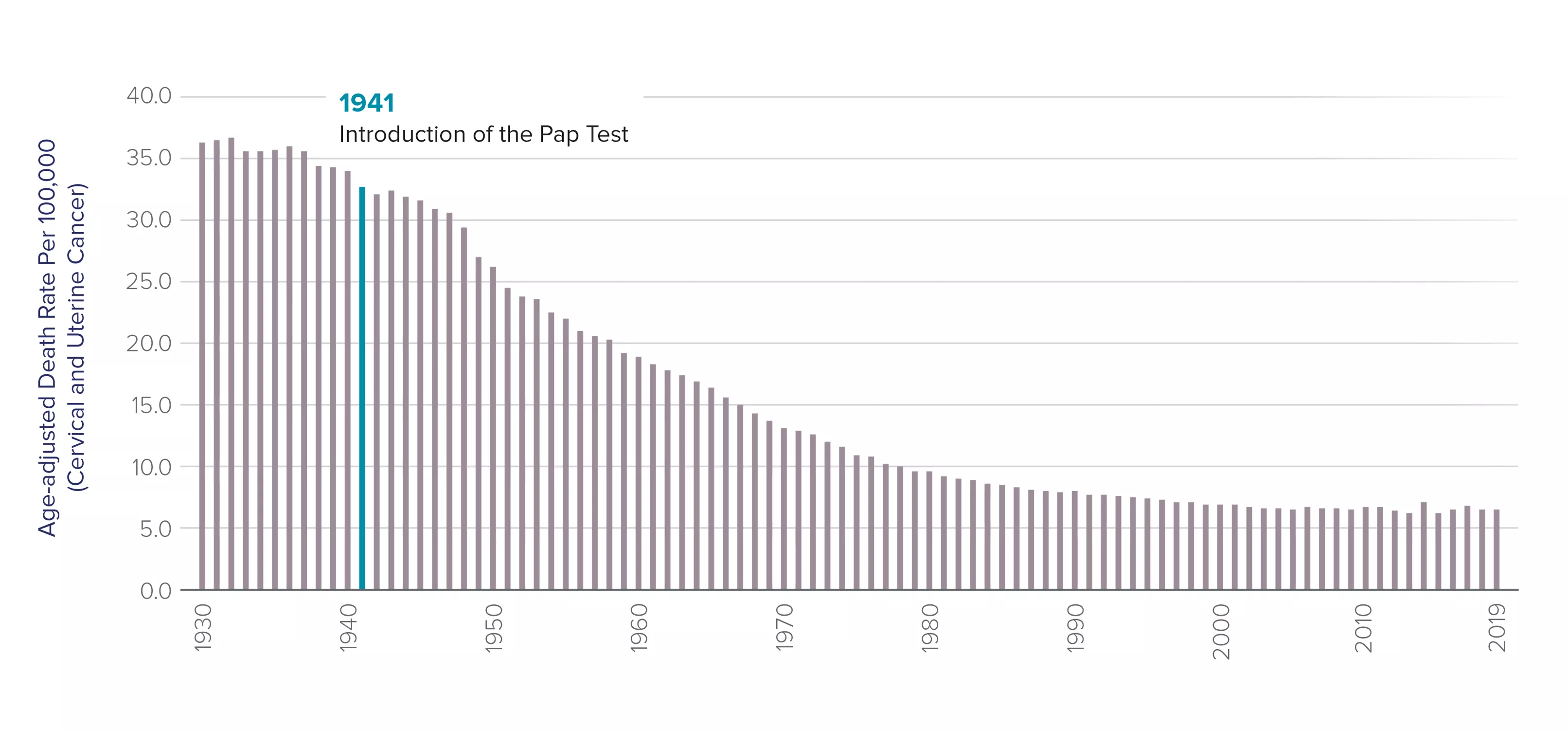
The Pap test has been the most successful cancer screening program in history.4
The rate of cervical cancer, which was a leading cause of death among women, has fallen by more than 70 percent since the Pap test was introduced over 80 years ago.5 Previously, cervical cancer was the leading cause of cancer death in women, but now it is the fifteenth most frequent.
Reducing cervical cancer incidence requires a comprehensive strategy.
Such a strategy will include screening options that take into account disparities that underserved women face. This strategy should focus on reducing preventive screening gaps by:
Implementing provider and patient education
Providing access to vaccinations
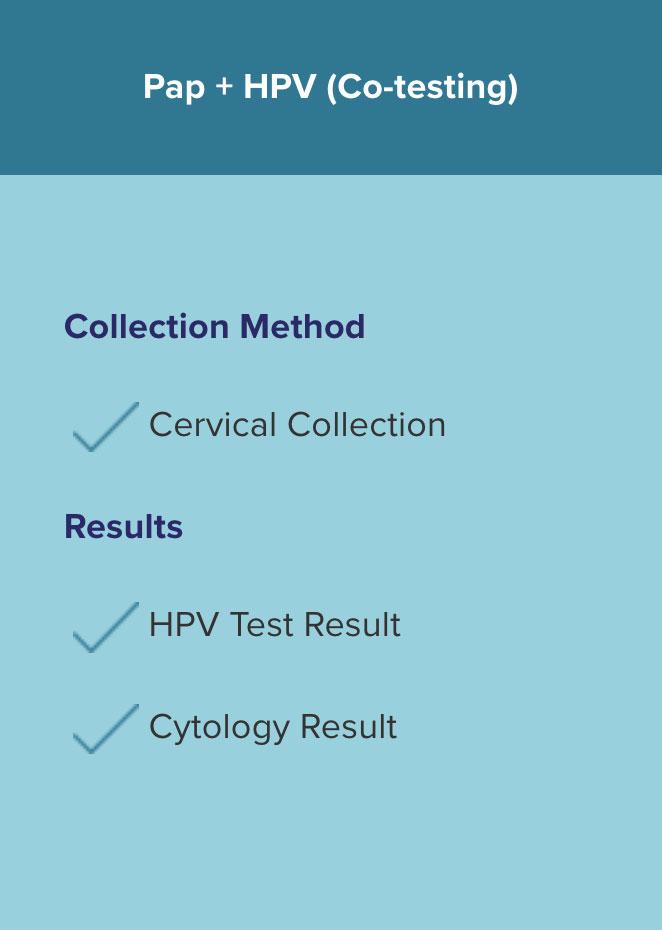
Offering Pap + HPV (co-testing)
ACOG, ASCCP, SGO and USPSTF guidelines recommend:
For women ages
21-29 years6
Screening with cervical cytology alone every 3 years is recommended.
For women ages
30-65 years old*6
Co-testing with cervical cytology and high-risk HPV testing every 5 years is recommended.
For women ages
65 years and older6
Do not require screening after adequate prior negative screening results.
The Preferred Choice in Pap Testing
- The ThinPrep Pap test was the first liquid-based cytology option in cervical cancer screening. In more than two decades the ThinPrep Pap test has contributed to a significant decline in cervical cancer rates.1,7
- More than 80% of the Pap Tests in the US are preformed with ThinPrep, with more than 1 billion tests preformed globally so far.8
- 90% of the Top US Best Hospitals for gynecology trust the ThinPrep Pap Test. 8,9
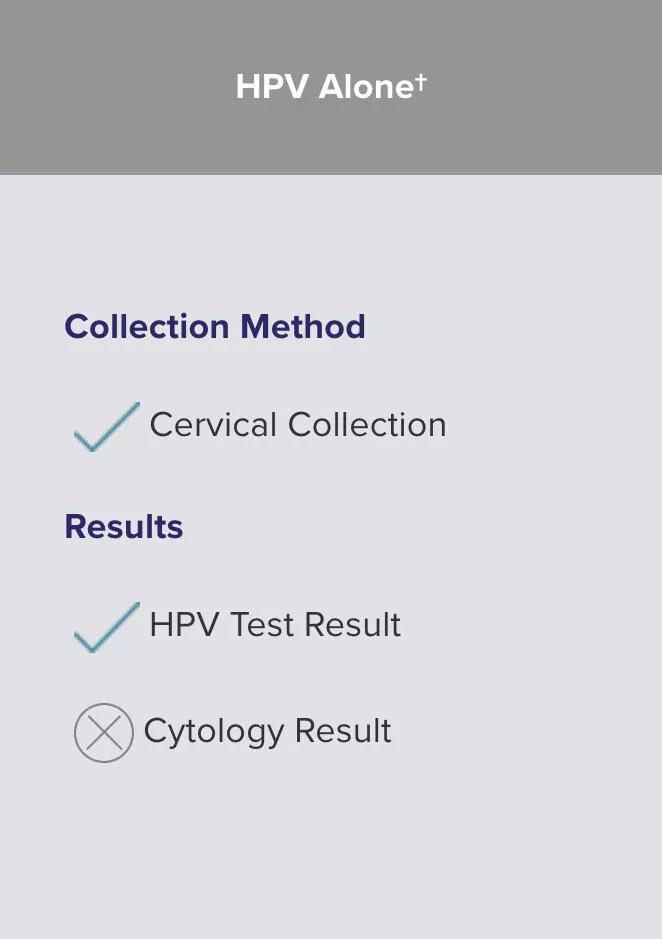
Regardless of the algorithm, the collection method is the same.
The difference is in the results — with HPV-Alone†, you will receive less information with the same collection. Samples may be collected in an FDA approved liquid-based cytology medium, such as ThinPrep® Pap Test.

A comprehensive list of FDA Approvals/Clearances for Ancillary Testing Needs:

-
ThinPrep® Pap Test
-
Aptima® HPV assay
-
Aptima® HPV 16 18/45 genotype assay
-
Aptima Combo 2® assay for CT/NG
-
Aptima® Trichomonas vaginalis assay
- cobas® HPV assay
- cobas® AMPLICOR CT/NG test
- Hybrid Capture 2 HPV DNA Test
- ProbeTec™ CT/GC Qx Amplified DNA assays
Testing out of the ThinPrep vial requires no cumbersome pre-treatment steps for similar workflow.1
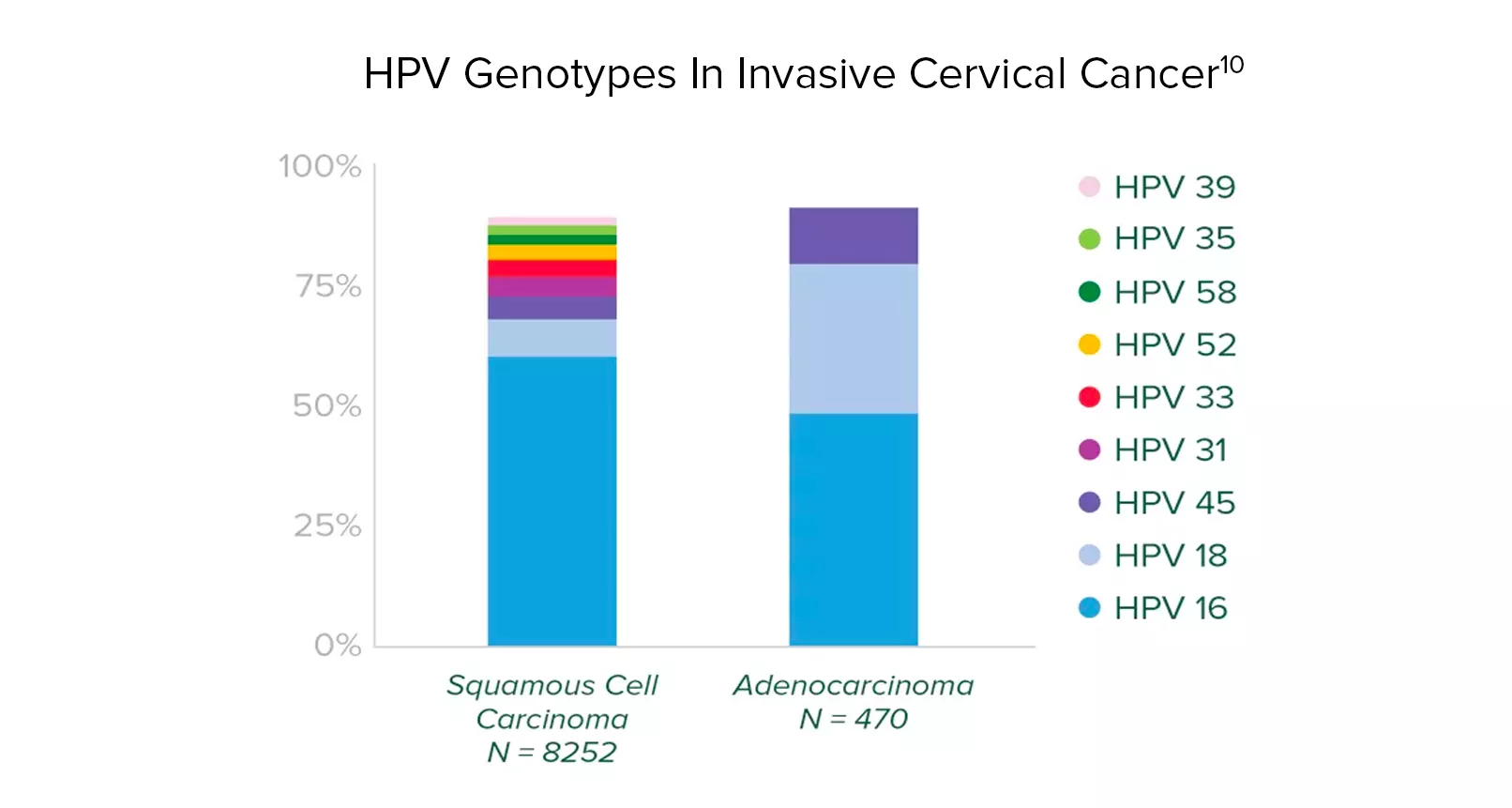
About HPV Type 45:
-
It is uncommon and only prevalent in 0.4% of women with normal cytology.10
-
It is the third most common HPV type in invasive cervical cancer.10,11
-
Adenocarcinoma is associated with types 16, 18 and 45.12,13
The Aptima HPV 16 18/45 genotype assay targets these genotypes. These genotypes identify more women at risk with minimal impact to colposcopy rates.10
HPV types 16, 18, and 45 are associated with up to
94%
of HPV-related cervical adenocarcinomas.10
Identify
Identify the presence and activity of high-risk HPV Infection.14,15
Superior specificity
Equivalent sensitivity to DNA based tests, with superior specificity.2,16,17
Next generation
Next generation genotyping.
Aptima® mRNA HPV testing: detects E6/E7 mRNA. The expression of E6/E7 is indicative of the HPV infections most likely to lead to disease.14,15 The Aptima® HPV assay maximizes the benefits of screening while minimizing the potential harm.
Studies have shown that while nearly all unvaccinated sexually active men and women will have HPV at some point in their lives, very few infections will progress to cancer.18
* Aptima HPV assay, Aptima HPV 16 18/45 Genotype assay, Cervista HPV HR test, Cervista HPV 16/18 test, Roche cobas HPV test and Hybrid Capture 2 HPV DNA test.
† A positive HPV screening result may lead to further evaluation with cytology and/or colposcopy.
References:
1. ThinPrep 2000 Processor. US instructions for use MAN-02624-001. Hologic, Inc.; 2021. 2. Aptima HPV assay. US package insert AW-12820-001. San Diego, CA: Hologic, Inc.; 2020. 3. Aptima HPV Genotype 16 18/45 assay. US package insert AW-12821-001. San Diego, CA: Hologic, Inc.; 2020. 4. American Cancer Society. The Pap (Papanicolaou) Test. American Cancer Society website. Last Revised January 3, 2020. Accessed April 28, 2022. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/screening-tests/pap-test.html. 5. American Cancer Society. Cancer Statistics Center. Published 2018. Accessed May 13, 2022. https://cancerstatisticscenter.cancer.org/#!/cancer-site/Cervix 6. American College of Obstetricians and Gynecologist. Women’s Health Care Physicians. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines. Released April 2021. Accessed May 3, 2022 7. SEER21 Delay-Adjusted Incidence rate Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 21 Registries, Nov 2019 Sub (1975-2017). 8. Hologic, Inc. Data on file. 9. US News & World Report. Best Hospitals for Adult Gynecology. US News & World Report website. Published 2021. Accessed April 26, 2022. https://health.usnews.com/best-hospitals/rankings/gynecology. 10. de Sanjose S, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048-1056..11. Hopenhayn C, et al. Prevalence of Human Papillomavirus Types in Invasive Cervical Cancers From 7 US Cancer Registries Before Vaccine Introduction. J of Low Genit Tract Dis. 2014;18(3):182-9. 12. Tjalma WA, et al. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int. J. Cancer. 2013;132(4):854–867. 13. Guan P, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Intl. J Cancer. 2012;131(10):2349-2359. 14. Tinelli A, et al. HPV viral activity by mRNA-HPV molecular analysis to screen the transforming infections in precancer cervical lesions. Curr Pharm Biotechnol. 2009;10(8):767-771. 15. Cuschieri K, et al. Human papillomavirus type specific DNA and RNA persistence–implications for cervical disease progression and monitoring. J Med Virol. 2004;73(1):65-70. doi:10.1002/jmv.20062. 16. Iftner T, et al. Head-to-head comparison of the RNA-based Aptima human papillomavirus (HPV) assay and the DNA-based hybrid capture 2 HPV test in a routine screening population of women aged 30 to 60 years in Germany. J Clin Microbiol. 2015;53(8):2509-2516. 17. Cuzick J, et al. Comparing the performance of six human papillomavirus tests in a screening population. Br J Cancer. 2013;108:908-913. doi:10.1038bjc.2013.22. 18. CDC. Genital HPV Infection - CDC Fact Sheet. Centers for Disease Control and Prevention website. Published July 2017. Accessed April 28, 2022.https://www.cdc.gov/std/hpv/HPV-FS-July-2017.pdf.
Safety Data Sheets
Package Inserts
Peer Reviewed Publications
Ashfaq R, Gibbons D, Vela C, Saboorian MH, Iliya F. ThinPrep Pap Test accuracy for glandular disease. Acta Cytol. 1999;43:81-5.
Bai H, Sung CJ, Steinhoff MM. ThinPrep Pap Test promotes detection of glandular lesions of the endocervix. Diagn Cytopathol. 2000;23:19-22.
Baker JJ. Conventional and liquid-based cervicovaginal cytology: a comparison study with clinical and histologic follow-up. Diagn Cytopathol. 2002;27(3):185-8.
Belinson J, Qiao YL, Pretorius R, Zhang WH, Elson P, Li L, Pan QJ, Fischer C, Lorincz A, Zahniser D. Shanxi Province cervical cancer screening study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol. 2001;83:439-44.
Belinson J, Qiao Y, Pretorius R, Zhang W, Keaton K, Elson P, Fischer C, Lorincz A, Zahniser D, Wilbur D, Pan Q, Li L, Biscotti C, Dawson A, Li A, Wu L, Ling Y, Ma CP, Yang YP. Prevalence of cervical cancer and feasibility of screening in rural China: a pilot study for the Shanxi Province Cervical Cancer Screening Study. Int J Gynecol Cancer. 1999;9:411-7.
Bolick DR, Hellman DJ. Laboratory implementation and efficacy assessment of ThinPrep cervical cancer screening system. Acta Cytol. 1998;42:209-13.
Carpenter AB, Davey DD. ThinPrep Pap Test: performance and biopsy follow-up in a university hospital. Cancer (Cancer Cytopathol). 1999;87(3):105-12.
Cheung AN, Szeto EF, Leung BSY, Khoo US, Ng AWY. Liquid-based cytology and conventional cervical smears: a comparison study in an Asian screening population. Cancer (Cancer Cytopathol). 2003;99(6):331-5.
Corkill M, Knapp D, Martin J, Hutchinson ML. Specimen adequacy of ThinPrep sample preparations in a direct-to-vial study. Acta Cytol. 1997;41:39-44.
Diaz-Rosario LA, Kabawat SE. Performance of a fluid-based, thin-layer Papanicolaou smear method in the clinical setting of an independent laboratory and an outpatient screening population in New England. Arch Pathol Lab Med. 1999;123(9):817-21.
Duggan MA, Khalil M, Brasher PMA, Nation JG. Comparative study of the ThinPrep Pap Test and conventional cytology results in a Canadian cohort. Cytopathology. 2006;17(2):73-81.
Dupree WB, Suprun HZ, Beckwith DG, Shane JJ, Lucente V. The promise and risk of a new technology: the Lehigh Valley Hospital’s experience with liquid-based cervical cytology. Cancer (Cancer Cytopathol). 1998;84:202-7.
Ferris DG, Heidemann NL, Litaker MS, Crosby JH, Macfee MS. The efficacy of liquid-based cervical cytology using direct-to-vial sample collection. J Fam Pract. 2000;49(11):1005-11.
Guidos BJ, Selvaggi SM. Use of the ThinPrep Pap Test in clinical practice. Diagn Cytopathol. 1999;20:70-3.
Guo M, Hu L, Martin L, Liu S, Baliga M, Hughson M. Accuracy of liquid based pap tests: comparison of concurrent liquid-based tests and cervical biopsies on 782 women with previously abnormal pap smears. Acta Cytol. 2005;49(2):132-8.
Hatch K, Sheets E, Kennedy A, Ferris D, Darragh T, Twiggs L. Multicenter direct to vial evaluation of a liquid-based pap test. J Low Genit Tract Dis. 2004;8(4):308-12.
Henderson S, Stevens M, Walker T. Rapid review of liquid-based smears as a quality control measure. Diagn Cytopathol. 2004;31(3):141-6.
Hoelund B. Implementation of liquid-based cytology in the screening programme against cervical cancer in the County of Funen, Denmark and status for the first year. Cytopathology. 2003;14(5):269-74.
Johnson JE, Rahemtulla A. Endocervical glandular neoplasia and its mimics in ThinPrep Pap tests. A descriptive study. Acta Cytol. 1999;43(3):369-75.
Limaye A, Connor AJ, Huang X, Luff R. Comparative analysis of conventional Papanicolaou tests and a fluid-based thin-layer method. Arch Pathol Lab Med. 2003;127(2):200-4.
Liverani M, De Paola F, Danesi S, Fedriga R, Dalbuoni V, Agnoletti R, Amadori AR, Mirra F, Bonoli W, Saragoni L. [Applicative aspects of liquid-based cytology in cervical cancer screening]. Pathologica. 2006;98(6):629-34. [article in Italian]
Nam JH, Kim HS, Lee JS, Choi HS, Min KW, Park CS. A comparison of modified MonoPrep2 of liquid-based cytology with ThinPrep Pap test. Gynecol Oncol. 2004;94(3):693-8.
Nance KV. Evolution of pap testing at a community hospital – a ten year experience. Diagn Cytopathol. 2007;35(3):148-53.
Negri G, Menia E, Egarter-Vigl E, Vittadello F, Mian C. ThinPrep versus conventional Papanicolaou smear in the cytologic follow-up with women with equivocal cervical smears. Cancer. 2003; 99(6):342-5.
Obwegeser JH, Brack S. Does liquid-based technology really improve detection of cervical neoplasia? A prospective, randomized trial comparing the ThinPrep Pap Test with the conventional Pap test, including follow-up of HSIL cases. Acta Cytol. 2001;45:709-14.
Papillo J, Zarka MA, St. John TL. Evaluation of the ThinPrep Pap Test in clinical practice: a seven-month 16,314-case experience in northern Vermont. Acta Cytol. 1998;42:204-8.
Schledermann D, Ejersbo D, Hoelund B. Improvement of diagnostic accuracy and screening conditions with liquid-based cytology. Diagn Cytopathol. 2006;34(11):780-5.
Schledermann D, Ejersbo D, Hoelund B. Significance of atypia in conventional Papanicolaou smears and liquid-based cytology: a follow-up study. Cytopathology. 2004;15(3):148-53.
Schorge JO, Saboorian MH, Hynan L, et al. ThinPrep detection of endometrial adenocarcinoma: a retrospective cohort study. Cancer Cytopathol. 2002;96:338-43.
Selvaggi SM. Cytologic features of high-grade squamous intraepithelial lesions involving endocervical glands on ThinPrep cytology. Diagn Cytopathol. 2002;26(3):181-5.
Selvaggi SM, Guidos BJ. Endocervical component: is it a determinant of specimen adequacy? Diagn Cytopathol. 2002;26(1):53-5.
Selvaggi SM, Guidos BJ. Specimen adequacy and the ThinPrep Pap Test: the endocervical component. Diagn Cytopathol. 2000;23(1):23-6.
Strander B, Andersson-Ellström A, Milson I, Rådberg T, Ryd W. Liquid-based cytology versus conventional Papanicolaou smear in an organized screening program. Cancer (Cancer Cytopathol). 2007; Aug 27 Epub ahead of print.
Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, Wright TC Jr. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2005;118(4):957-62.
Weintraub J, Morabia A. Efficacy of a liquid-based thin layer method for cervical cancer screening in a population with a low incidence of cervical cancer. Diagn Cytopathol. 2000;22(1):52-9.
Williams ARW. Liquid-based cytology and conventional smears compared over two 12-month periods. Cytopathology. 2006;17(2):82-5.
Yeoh GPS, Chan KW, Lauder I, Lam MB. Evaluation of the ThinPrep Papanicolaou Test in clinical practice: six-month study of 16, 541 cases with histological correlation in 220 cases. Hong Kong Med J. 1999;5:233-9.
Biscotti CV, O’Brien DL, Gero MA, Gramlich TL, Kennedy AW, Easley KA. Thin-layer Pap test vs. conventional Pap smear: analysis of 400 split samples. J Reprod Med. 2002;47(1):9-13.
Chhieng DC, Talley LI, Roberson J, Gatscha RM, Jhala NC, Elgert PA. Interobserver variability: comparison between liquid-based and conventional preparations in gynecologic oncology. Cancer (Cancer Cytopathol). 2002;96(2):67-73.
Clark SB, Dawson AE. Invasive squamous-cell carcinoma in ThinPrep specimens: diagnostic clues in the cellular pattern. Diagn Cytopathol. 2002;26(1):1-4.
Corkill M, Knapp D, Hutchinson ML. Improved accuracy for cervical cytology with the ThinPrep method and the endocervical brush-spatula collection procedure. J Lower Gen Tract Dis. 1998;2:12-16.
Cremer M, de LasCasas L, Kurtycz DFI, Schink J. Liquid-based cytology specimen studies to screen for cervical dysplasia in rural El Salvador. Int J Gynecol & Obstet. 2005;90(2):167-70.
Ferreccio C, Bratti M, Sherman M, Herrero R, Wacholder S, Hildesheim A, Burk RD, Hutchinson M, Alfaro M, Greenberg M, Morales J, Rodriguez A, Schussler J, Eklund C, Marshall G, Schiffman M. A comparison of single and combined visual, cytologic, and virologic tests as screening strategies in a region at high risk of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:815-23.
Grace A, McBrearty P, Troost S, Thornhill M, Kay E, Leader M. Comparative study: conventional cervical and ThinPrep Pap Tests in a routine clinical setting. Cytopathology. 2002;13:200-5.
Harkness CB, Theofrastous JP, Ibrahim SN, Galvin SL, Lawrence HC. Papanicolaou and thin-layer cervical cytology with colposcopic biopsy control: a comparison. J Reprod Med. 2003;48(9):681-6.
Haroon S, Samayoa L, Witzke D, Davey D. Reproducibility of cervicovaginal ThinPrep cellularity assessment. Diagn Cytopathol. 2002;26(1):19-21.
Hussein T, Desai M, Tomlinson A, Kitchener HC. The comparative diagnostic accuracy of conventional and liquid-based cytology in a colposcopic setting. BJOG. 2005;112(11):1542-6.
Hutchinson ML, Zahniser DJ, Sherman ME, Herrero R, Alfaro M, Bratti MC, Hildesheim A, Lorincz AT, Greenberg MD, Morales J, Schiffman M. Utility of liquid-based cytology for cervical carcinoma screening: results of a population-based study conducted in a region of Costa Rica with a high incidence of cervical carcinoma. Cancer (Cancer Cytopathol). 1999;87(2):48-55.
Inhorn SL, Wilbur D, Zahniser D, Linder J. Validation of the ThinPrep Pap Test for cervical cancer diagnosis. J Low Gen Tract Dis. 1998;2:208-12.
Lee KR, Darragh TM, Joste NE, Krane JF, Sherman ME, Hurley LB, Allred EM, Manos MM. Atypical glandular cells of undetermined significance (AGUS): Interobserver reproducibility in cervical smears and corresponding thin-layer preparations. Am J Clin Pathol. 2002;117:96-102.
Lee KR, Ashfaq R, Birdsong GG, Corkill ME, McIntosh KM, Inhorn SL. Comparison of conventional Papanicolaou smears and a fluid-based, thin-layer system for cervical cancer screening. Obstet Gynecol. 1997;90:278-84.
Linder J, Zahniser DJ. ThinPrep Papanicolaou testing to reduce false-negative cervical cytology. Arch Path Lab Med. 1998;122:139-44.
Luthra UK, Chishti M, Dey P, Jolly SV, Abdulla M, Das DK, Sugathan TN, Ajrawi MT, George J, George SS, Aziz AA, Al-Juwaiser A, Karim FA, Mallik MK, Sheikh ZA, Khan S. Performance of monolayered cervical smears in a gynecology outpatient setting in Kuwait. Acta Cytol. 2002; 46(2):303-10.
Malle D, Pateinakis P, Chakka E, Destouni C. Experience with a thin-layer, liquid-based cervical cytologic screening method. Acta Cytol. 2003;47(2):129-34.
Menezes GA, Wakely PE, Stripe DM, Nuovo GJ. Increased incidence of atypical Papanicolaou tests from ThinPreps of postmenopausal women receiving hormone replacement therapy. Cancer (Cancer Cytopathol). 2001;93(6):357-63.
Monsonego J, Autillo-Touati A, Bergeron C, Dachez R, Liaras J, Saurel J, Zerat L, Chatelain P, Mottot C. Liquid-based cytology for primary cervical cancer screening: a multi-centre study. Br J Cancer. 2001;84(3):360-6.
Park IA, Lee SN, Chae SW, Park KH, Kim JW, Lee HP. Comparing the accuracy of ThinPrep Pap tests and conventional Papanicolaou smears on the basis of the histologic diagnosis: a clinical study of women with cervical abnormalities. Acta Cytol. 2001;45(4):525-31.
Rinas AC, Mittman BW, Van Le L, Hartmann K, Cayless J, Singh HK. Split-sample analysis of discarded cells from liquid-based pap smear sampling devices. Acta Cytol. 2006;50(1)55-62.
Ring M, Bolger N, O’Donnell M, Malkin A, Bermingham N, Akpan E, Mulcahy F, Turner NJ, Griffin M, O’Leary JJ. Evaluation of liquid-based cytology in cervical screening of high-risk populations: a split study of colposcopy and genito-urinary medicine populations. Cytopathology. 2002;13:152-9.
Roberts J, Thurloe J. Comparative sensitivities of the ThinPrep and Papanicolaou smear for adenocarcinoma in situ (AIS) and combined AIS/high-grade squamous intraepithelial lesion (HSIL): comparison with HSIL. Cancer (Cancer Cytopathol). 2007: October 29 Epub ahead of print.
Roberts JM, Thurloe JK, Bowditch RC, Humcevic J, Laverty CR. Comparison of ThinPrep and Pap smear in relation to prediction of adenocarcinoma in situ. Acta Cytol. 1999; 43(1):74-80.
Roberts JM, Gurley AM, Thurloe JK, Bowditch R, Laverty CR. Evaluation of the ThinPrep Pap Test as an adjunct to the conventional Pap smear. Med J Aust. 1997;167:466-7.
Rooney CM, Hopkins MP, Oza R, Nelson K, Alford W. The efficacy of the ThinPrep Pap preparation versus conventional means of cervical cancer screening. J Pelvic Med Surg. 2004;10(1):31-5.
Sherman ME, Mendoza M, Lee KR, Ashfaq R, Birdsong GG, Corkill ME, McIntosh KM, Inhorn SL, Zahniser DJ, Baber G, Barber C, Stoler MH. Performance of liquid-based, thin-layer cervical cytology: correlation with reference diagnoses and human papillomavirus testing. Mod Pathol. 1998;11:837-43.
Sherman ME, Schiffman MH, Lorincz AT, Herrero R, Hutchinson ML, Bratti C, Zahniser D, Morales J, Hildesheim A, Helgesen K, Kelly D, Alfaro M, Mena F, Balmaceda I, Mango L, Greenberg M. Cervical specimens collected in liquid buffer are suitable for both cytologic screening and ancillary human papillomavirus testing. Cancer. 1997;81:89-97.
Shield PW, Nolan GR, Phillips GE, Cummings MC. Improving cervical cytology screening in a remote, high risk population. Med J Aust. 1999;170(6):255-8.
Song LH, Goh EST, Phang LC, Poh WT, Tay SK. Technical aspect of ThinPrep. Singapore Med J. 2000;41(12):575-8.
Tuncer ZS, Basaran M, Sezgin Y, Firat P, Mocan Kuzey G. Clinical results of a split sample liquid-based cytology (ThinPrep) study of 4,322 patients in a Turkish institution. Eur J Gynaecol Oncol. 2005;26(6):646-8.
Wang TY, Chen HS, Yang YC, Tsou MC. Comparison of fluid-based, thin-layer processing and conventional Papanicolaou methods for uterine cervical cytology. J Formos Med Assoc. 1999;98(7):500-5.
Zhu J, Norman I, Elfren K, Gaberi V, Hagmar B, Hjerpe A, Andersson S. A comparison of liquid-based cytology and Pap smear as a screening method for cervical cancer. Oncol Reports. 2007;18:157-60.
Abulafia O, Pezzullo JC, Sherer DM. Performance of the ThinPrep liquid-based cervical cytology in comparison with conventionally prepared Papanicolaou smears: a quantitative survey. Gynecol Oncol. 2003;90(1):137-44.
Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cytology. Obstet Gynecol. 2008;111:167-77.
Bernstein SJ. Sanchez-Ramos, L, Ndubisi B. Liquid-based cervical cytologic smear study and conventional Papanicolaou smears: a metaanalysis of prospective studies comparing cytologic diagnosis and sample adequacy. Am J Obstet Gynecol. 2001;185:308-17.
Davey E, Barratt A, Irwig L, Chan SF, Macaskill P, Mannes P, Saville AM. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122-32.
Klinkhamer P, Meerding W, Rosier P, Hanselaar A. Liquid-based cervical cytology: a review of the literature with methods of evidence-based medicine. Cancer (Cancer Cytopathol). 2003;99(5):263-71.
Ciesla MC, Guidos BJ, Selvaggi SM. Cytomorphology of small-cell (neuroendocrine) carcinoma on ThinPrep(R) cytology as compared to conventional smears. Diagn Cytopathol. 2001;24(1):46-52.
Hoerl HD et al: Exfoliative cytology of primary poorly differentiated (small-cell) neuroendocrine carcinoma of the uterine cervix in ThinPrep material. A case report. Diagn Cytopathol. 2000; 23(1):14-8.
Rahimpanah F, Reid RI. Fallopian tube carcinoma detected by ThinPrep cytology smear. Med J Aust. 2000;17(1):38.
Selvaggi S, Guidos B. Immature teratoma of the ovary on fluid cytology. Diagn Cytopathol. 2001;25(6):411-4.
Andy C, Turner LF, Neher JO. Is the ThinPrep better than conventional Pap smear at detecting cervical cancer? J Fam Pract. 2004;53(4):313-5.
Austin RM, Ramzy I. Increased detection of epithelial cell abnormalities by liquid-based gynecologic cytology preparations. A review of accumulated data. Acta Cytol. 1998;42(1):178-84.
Broadstock M. Liquid-based cytology: an alternative international view. Cytopathology. 2001;12:141-3.
Farnsworth A. Liquid-based cytology: an Australian experience. Cytopathology. 2003;14:48-52.
Hartman KE, Nanda K, Hall S, Myers E. Technologic advances for evaluation of cervical cytology: is newer better? Obstet Gynecol Surv. 2001;56(12):765-74.
Kurtycz D, Hoerl H. Thinlayer technology: tempered enthusiasm. Diagn Cytopathol. 2000;23(1):1-5.
Linder J. Recent advances in thin-layer technology. Diagn Cytopathol. 1998;18(1):24-32.
Linder J, Zahniser D. The ThinPrep Pap Test: a review of clinical studies. Acta Cytol. 1997;41(1):30-8.
Myers ER. Improving the sensitivity of cervical cytology: what are the issues? Am J Manag Care. 2000;6:838-40.
Nanda K et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810-9.
Obwegeser J, Schneider V. Thin-layer cervical cytology: a new meta-analysis. Lancet. 2006;367:88-9.
Perlman SE: Pap smears. Screening, interpretation, treatment. Adolesc Med. 1999;10(2):243-54.
Rappaport KM, Forrest CB, Holtzman NA. Adoption of liquid-based cervical cancer screening tests by family physicians and gynecologists. Health Serv Res. 2004;39(4 Pt 1):927-47.
Renshaw AA, Dubray-Benstein B, Cobb CJ, Loano RL, Neal MH, Prey M, Schulty MA; Gynecologic Cytology Committee, College of American Pathologists. Cytologic features of squamous cell carcinoma in ThinPrep slides: evaluation of cases that performed poorly versus those that performed well in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004;128(4):403-5.
Renshaw AA, Young NA, Birdsong GG, Styer PE, Davey DD, Mody DR, Colgan TJ. Comparison of performance of conventional and ThinPrep gynecologic preparations in the College of American Pathologists Gynecologic Cytology Program. Arch Pathol Lab Med. 2004;128(1):17-22.
Renshaw AA, Schulte MA, Plott E, Dubray-Benstein B, Cobb CJ, Lozano RL, Neal MH, Hughes JH, Young NA, Prey M. Cytologic features of high-grade squamous intraepithelial lesion in ThinPrep Papanicolaou test slides: comparison of cases that performed poorly with those that performed well in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004;128(7):746-8.
Soler ME, Blumenthal PD. New technologies in cervical cancer screening. Curr Opin Oncol. 2000;12:460-5.
Spitzer M. Cervical screening adjuncts: recent advances. Am J Obstet Gynecol. 1998;179(2):544-56.
Stoler MH. Advances in cervical screening technology. Mod Pathol. 2000;12(3):275-84.
Wilson PO. Liquid-based cytology: the evidence is out there? Cytopathology. 2006;17(2):57-9.
Akpolat I, Smith DA, Ramzy I, Chirala M, Mody DR. The utility of p16INK4a and Ki-67 staining on cell blocks prepared from residual thin-layer cervicovaginal material. Cancer. 2004;102(3):142-9.
Al-Awadhi R, Van Noorden S, Coleman DV. Evaluation of NMP179 for the detection of squamous intraepithelial neoplasia in ThinPrep cervical slides using a combination of double immunostaining and morphometric methods of analysis. Diagn Cytopathol. 2004;31(3):135-40.
Bianchi A, Moret F, Desrues JM, Champenois T, Dervaux Y, Desvouas O, Oursin A, Quinzat D, Dachez R, Bathelier C, Ronsin C. PreservCyt transport medium used for the ThinPrep Pap test is a suitable medium for detection of Chlamydia trachomatis by the COBAS Amplicor CT/NG test: results of a preliminary study and future implications. J Clin Microbiol. 2002;40(5):1749-54.
Bibbo M, DeCecco J, Kovatich AJ. P16INK4a as an adjunct test in liquid-based cytology. Anal Quant Cytol Histol. 2003;25(1):8-11.
Bibbo M, Klump WJ, DeCecco J, Kovatich AJ. Procedure of immunocytochemical detection of P16INK4a antigen in thin-layer, liquid-based specimens. Acta Cytol. 2002;46(1):25-9.
Bollman M, Varani AD, Griefingholt H, Bankfalvi A, Callenberg H, Speigh N, Schmitt C, Bollman R. Predicting treatment outcome in cervical diseases using liquid-based cytology, dynamic HPV genotyping and DNA cytometry. Anticancer Res. 2006;26(2B):1439-46.
Bollmann R, Mehes G, Torka R, Speich N, Schmitt C, Bollmann M. Determination of features indicating progression in atypical squamous cells with undetermined significance: human papillomavirus typing and DNA ploidy analysis from liquid-based cytologic samples. Cancer (Cancer Cytopathol). 2003;99(2):113-7.
Bollmann R, Mehes G, Torka R, Speich N, Schmitt C, Bollmann M. Human papillomavirus typing and DNA ploidy determination of squamous intraepithelial lesions in liquid-based cytologic samples. Cancer (Cancer Cytopathol). 2003;99(1):57-62.
Carozzi FM, Del Mistro A, Confortini M, Sani C, Puliti D, Trevisan R, De Marco L, Tos AG, Girlando S, Palma PD, Pellegrini A, Schiboni ML, Crucitti P, Pierotti P, Vignato A, Ronco G. Reproducibility of HPV DNA testing by Hybrid Capture 2 in a screening setting. Am J Clin Pathol. 2005;124(5):716-21.
Carydis VB, Walker T, Wing A, Colgan T. Utility of p16ink4a Immunocytochemistry in liquid-based cytology specimens from women treated for high-grade squamous intraepithelial lesions. Acta Cytol. 2007;51:517-22.
Castle PE, Solomon D, Hildesheim A, Herrero R, Bratti MC, Sherman ME, Rodriquez AC, Alfaro M, Hutchinson ML, Dunn ST, Kuypers J, Schiffman M. Stability of archived liquid-based cervical cytological specimens. Cancer (Cancer Cytopathol). 2003;99(2):89-96.
Chernesky M, Jang D, Portillo E, Chong S, Smieja M, Luinstra K, Petrich A, MacRitchie C, Ewert R, Hayhoe B, Sarabia A, Thompson F. Ability of APTIMA, AMPLICOR, and ProbeTec assays to detect Chlamydia trachomatis and Neisseria gonorrhpeae in PreservCyt ThinPrep liquid based pap samples. J Clin Microbiol. 2007;May 30 [Epub ahead of print].
Cheung AN, Chiu PM, Tsun KL, Khoo US, Leung BS, Ngan HY. Chromosome in situ hybridisation, Ki-67, and telomerase immunocytochemistry in liquid based cervical cytology. J Clin Pathol. 2004;57(7):721-7.
Clavel C, Masure M, Bory JP, Putaud I, Mangeonjean C, Lorenzato M, Nazeyrollas P, Gabriel R, Quereux C, Birembault P. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7,932 women. Br J Cancer. 2001;89(12):1616-23.
Crum CP, Genest DR, Krane JF, Hogan C, Sun D, Bellerose B, Kostopoulou E, Lee KR. Subclassifying atypical squamous cells in ThinPrep cervical cytology correlates with detection of high-risk human papillomavirus DNA. Am J Clin Pathol. 1999;112:384-90.
Cubie HA, Moore C, Waller M, Moss S; National Cervical Screening Committee LBC/HPV Pilot Steering Group. The development of a quality assurance programme for HPV testing within the UK NHS cervical screening LBC/HPV studies. J Clin Virol. 2005;33(4):287-92.
Cubie HA, Seagar AL, McGoogan E, Whitehead J, Brass A, Arends MJ, Whitley MW. Rapid real time PCR to distinguish between high risk human papillomavirus types 16 and 18. J Clin Pathol. 2001;54:24-9.
Dallenbach-Hellweg G, Trunk MJ, von Knebel Doeberitz M. Traditional and new molecular methods for early detection of cervical cancer. Arch Pathol Lab Med. 2004;66(5):35-9.
Dehn D, Torkko KC, Shroyer KR. Human papillomavirus testing and molecular markers of cervical dysplasia and carcimona. Cancer (Cancer Cytopathol). 2007;111(1):1-14.
Dimulescu I, Unger ER, Lee DR, Reeves WC, Vernon SD. Characterization of RNA in cytologic samples preserved in a methanol-based collection solution. Mol Diagn. 1998;3(2):67-71.
Duncan L, Jacob S, Hubbard E. Evaluation of p16INKa as a diagnostic tool in the triage of Pap smears demonstrating atypical squamous cells of undetermined significance. Cancer (Cancer Cytopathol). 2008;January 10 [EPub ahead of print].
Eltoum IA, Chhieng DC, Crowe R, Roberson J, Jin G, Broker T. Significance and possible causes of false-negative results of reflex human papillomavirus infection testing. Cancer (Cancer Cytopathol). 2007;111(3):154-9.
Eltoum IA, Chhieng DC, Roberson J, McMillon D, Partridge EE. Reflex human papilloma virus infection testing detects the same proportion of cervical intraepithelial neoplasia grade 2-3 in young versus elderly women. Cancer (Cancer Cytopathol). 2005;105(4):194-8.
Evans MF, Adamson CS, Papillo JL, St John TL, Leiman G, Cooper K. Distribution of human papillomavirus types in ThinPrep Papanicolaou tests classified according to the Bethesda 2001 terminology and correlations with patient age and biopsy outcomes. Cancer. 2006;106((5):1054-64.
Feng J, Husain M. Reflex high-risk human papillomavirus DNA testing (Hybrid Capture 2) of bloody ThinPrep specimens with atypical squamous cells of undetermined significance interpretation. Cancer (Cancer Cytopathol). 2005;105(5):452-6.
Feng J, Husain M. Outcomes of women with atypical squamous cells of undetermined significance and high-risk human papillomavirus DNA. Acta Cytol. 2007;51:730-4.
Ferenczy A, Franco E, Arseneau J, Wright TC Jr, Richart RM. Diagnostic performance of Hybrid Capture human papillomavirus deoxyribonucleic acid assay combined with liquid-based cytologic study. Am J Obstet Gynecol. 1996;175(3 Pt.1):651-6.
Ferris DG, Schiffman M, Litaker MS, for the Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) group. Cervicography for triage of women with mildly abnormal cervical cytology results. Am J Obstet Gynecol. 2001;185(4):939-43.
Fiel-Gan MD, Villamil CF, Mandavilli SR, Ludwig ME, Tsongalis GJ. Rapid detection of HSV from cytologic specimens collected into ThinPrep fixative. Acta Cytol. 1999;43:1034-1038.
Geng XX, Ma R, Zhu L, Gao QY. ThinPrep cytology and high-risk human papillomavirus detection on evaluating the efficacy of loop electrosurgical excision procedure in treatment of cervical intraepithelial neoplasia. Eur J Obstet Gynecol Reprod Biol. 2007; 131(2):242-3.
Ghanem KG, Koumans EH, Johnson RE, Sawyer MK, Papp JR, Unger ER, Black CM, Markowitz LE. Effect of specimen order on Chlamydia trachomatis and Neisseria gonorrhoeae test performance and adequacy of Papanicolaou smear. J Pediatr Adolesc Gynecol. 2006;19(1):23-30.
Hildebrandt EF, Lee JR, Crosby JH, Ferris DG, Anderson MG. Liquid-based Pap smears as a source of RNA for gene expression analysis. Appl Immunohistochem Mol Morphol. 2003;11(4):345-51.
Holladay EB, Logan S, Arnold J, Knesel B, Smith GD. A comparison of the clinical utility of p16(INK4a) immunolocalization with the presence of human papillomavirus by hybrid capture 2 for the detection of cervical dysplasia/neoplasia. Cancer (Cancer Cytopathol). 2006; 108(6):451-61.
Hong IS, Marshalleck J, Williams RH, Gaiter TE, Mielzynska-Lohnas I, Kim K. Comparative analysis of a liquid-based Pap test and concurrent HPV DNA assay of residual samples. Acta Cytol. 2002;46(5):828-34.
Hopwood J, Mallinson H, Hodgson E, Hull L. Liquid based cytology: examination of its potential in a chlamydia screening programme. Sex Transm Infect. 2004;80(5):371-3.
Hughes SA, Sun D, Gibson C, Bellerose B, Rushing L, Chen H, Harlow BL, Genest DR, Sheets ER, Crum CR. Managing atypical squamous cells of undetermined significance (ASCUS): human papillomavirus testing, ASCUS subtyping, or follow-up cytology? Am J Obstet Gynecol. 2002;186(3):396-403.
Inhorn SL, Wand PJ, Wright TC Jr, Hatch KD, Hallum J, Lentrichia BB. Chlamydia trachomatis and Pap testing from a single, fluid-based sample. A multicenter study. J Reprod Med. 2001;46(3):237-42.
Keegan H, Ryan F, Malkin A, Griffin M, Lambkin H. Chlamydia trachomatis detection in cervical PerservCyt specimens from an Irish urban female population. Cytopathology. 2007; December 17 [Epub ahead of print].
Keegan H, Boland C, Malkin A, Griffin M, Ryan F, Lambkin H. Comparison of DNA extraction from cervical cells collected in PreservCyt solution for the amplification of Chlamydia trachomatis. Cytopathology. 2005;16(2):82-7.
Kim JJ, Wright TC Jr, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002; 287(18):2382-90.
Knoepp S, Kuebler D, Wilbur D. Resolution of equivocal results with Hybrid Capture II high-risk HPV DNA test: a cytologic/histologic review of 191 cases. Diagn Mol Pathol. 2007;16:125-9.
Koumans EH, Black CM, Markowitz LA, Unger ER, Pierce A. Sawyer MK, Papp JR. Comparison of methods for detection of Chlamydia trachomatis and Neisseria gonorrhoeae using commercially available nucleic acid amplification tests and a liquid Pap smear medium. J Clin Microbiol. 2003;41(4):1507-11.
Lara-Torre E, Pinkerton JS. Accuracy of detection of Trichomonas vaginalis organisms on a liquid-based Papanicolaou smear. Am J Obstet Gynecol. 2003;188:354-6.
Lee HS, Kim KM, Kim SM, Choi YD, Nam JH, Park CS, Choi HS. Human papillomavirus genotyping using HPV DNA chip analysis in Korean women. Int J Gynecol Cancer. 2007;17:497-501.
Lee GY, Kim SM, Rim SY, Choi HS, Park CS, Nam JH. Human papillomavirus (HPV) genotyping by HPV DNA chip in cervical cancer and precancerous lesions. Int J Gynecol Cancer. 2005;15(1):81-7.
Lerma E, Quintana MJ, Quilez M, Esteva E, Carreras A, Bonfill X, Prat J, Calaf J. Effectiveness of liquid-based cytology and Papanicolaou tests in a low risk population. Acta Cytol. 2007;51:399-406.
Liman AK, Giampoli EJ, Bonfiglio TA. Should women with atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion, receive reflex human papillomavirus-DNA testing? Cancer (Cancer Cytopathol). 2005;105(6):457-60.
Lin WM, Ashfaq R, Michalopulos EA, Maitra A, Gazdar AF, Muller CY. Molecular Papanicolaou tests in the twenty-first century: molecular analyses with fluid-based Papanicolaou technology. Am J Obstet Gynecol. 2000;183:39-45.
Lu DW, Pirog EC, Zhu X, Wang HL, Pinto KR. Prevalence and typing HPV DNA in atypical squamous cells in pregnant women. Acta Cytol. 2003;47(6):1008-16.
Maksem JA, Bedrossian CW, Kurtycz D, Sewall S, Shalkham J, Dhanwada V, Lind H, Bibbo M, Weidmann J, Kane B, Shi Fu Y. Resolving ASCUS without recourse to HPV testing: manual reprocessing of residual automated liquid-based cytology (ALBC) material using manual liquid-based cytology (MLBC). Diagn Cytopathol. 2005;33(6):434-40.
Mandelblatt JS, Lawrence WF, Womack SM, Jacobson D, Yi B, Hwang Y, Gold K, Barter J, Shah K. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA. 2002;287(18):2372-81.
Manos MM, Kinney WK, Hurley LB, Sherman ME, Shieh-Ngai J, Kurman RJ, Ransley JE, Fetterman BJ, Hartinger JS, McIntosh KM, Pawlick GF, Hiatt RA. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-10.
Menezes G, Euscher E, Schwartz B, Catania F, Chancellor J, Nuovo GJ. Utility of the in situ detection of HPV in Pap smears diagnosed as within normal limits. Acta Cytol. 2001;45(6):919-26.
Meyer JL, Hanlin DW, Andersen BT, Rasmussen OF, Bisgaard K. Evaluation of p16(INK4a) expression in ThinPrep cervical specimens with the CINtec p16(INK4a) assay: correlation with biopsy follow-up results. Cancer. 2007; March 2; [Epub ahead of print].
Moore GD, Lear SC, Wills-Frank LA, Martin AW, Snyder JW, Helm CW. Differential expression of cdk Inhibitors p16, p21cip1, p27kip1, and Cyclin E in cervical cytological smears prepared by the ThinPrep method. Diagn Cytopathol. 2005;32(2):82-7.
Murphy N, Ring M, Heffron CC, King B, Killalea AG, Hughes C, Martin CM, McGuinness E, Sheils O, O’Leary JJ. p16INK4A, CDC6, and MCM5: predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J Clin Pathol. 2005;58(5):525-34.
Murphy N, Ring M, Killalea AG, Uhlmann V, O’Donovan M, Mulcahy F, Turner M, McGuinness E, Griffin M, Martin C, Sheils O, O’Leary JJ. P16INK4a as a marker for cervical dyskaryosis: CIN and cGIN in cervical biopsies and ThinPrep smears. J Clin Pathol. 2003;56(1):56-63.
Negri G, Rigo B, Vittadello F, Egarter-Vigl E, Mian C. Human papillomavirus typing with hybrid capture II on archived liquid-based cytologic specimens: is HPV typing always reproducible? Am J Clin Pathol. 2004;122(1):90-3.
Negri G, Gampenrieder J, Vig; EE, Haitel A, Menia E, Mian C. Human papillomavirus typing at large loop excision of the transformation zone of the cervix uteri. Anticancer Res. 2003;23(5b):4289-92.
Negri G, Egarter-Vigl E, Kasal A, Romano F, Haitel A, Mian C. P16INK4a is a useful marker for the diagnosis of adenocarcinoma of the cervix uteri and its precursors. Am J Surg Pathol. 2003;27(2):187-93.
Pan Q, Belinson JL, Li L, Pretorius RG, Qiao YL, Zhang WH, Zhang X, Wu LY, Rong SD, Sun YT. A thin-layer, liquid-based Pap test for mass screening in an area of China with a high incidence of cervical carcinoma: a cross-sectional, comparative study. Acta Cytol. 2003;47(1):45-50.
Pantanowitz L, Florence RR, Goulart RA, Otis CN. Trichomonas vaginalis P16 immunoreactivity in cervicovaginal Pap tests: a diagnostic pitfall. Diagn Cytopathol. 2005;33(3):210-3.
Peng Y, Wang HH. Impact of reflex HPV testing on interpretation and management of ThinPrep Pap tests. Diagn Cytopathol. 2006;34(8):585-8.
Peyton CL, Schiffman M, Lorincz AT, Hunt WC, Mielzynska I, Bratti C, Eaton S, Hildesheim A, Morera LA, Rodriguez AC, Herrero R, Sherman ME, Wheeler CM. Comparison of PCR and Hybrid Capture-based human papillomavirus detection systems using multiple cervical specimen collection strategies. J Clin Microbiol. 1998;36(11):3248-54.
Pinto KR, Lu DW, Rader JS, Davila RM. Double cervix with bilateral and synchronous HSIL associated with different high-risk HPV types. A case report. Acta Cytol. 2004;48(2):273-7.
Pirog EC, Erroll M, Harigopal M, Centeno BA. Comparison of human papillomavirus DNA prevalence in atypical squamous cells of undetermined significance subcategories as defined by the original Bethesda 1991 and the new Bethesda 2001 Systems. Arch Pathol Lab Med. 2004;128(5):527-32.
Powell N, Smith K, Fiander A. Recovery of human papillomavirus nucleic acids from liquid-based cytology media. J Virol Methods. 2006;137(1):58-62.
Qian DY, Cen JM, Wang D, Zeng RH, Lin AH, Shu YH, Hong DH, Huang ZH. Combining high-risk human papillomavirus DNA test and cytological test to detect early cervical dysplasia. Zhonghua Fu Chan Ke Za Zhi. 2006;41(1):34-7. [article in Chinese]
Quddus MR, Zhang S, Sung CJ, Liu F, Neves T, Struminsky J, Singer DB. Utility of HPV DNA detection in thin-layer, liquid-based tests with atypical squamous metaplasia. Acta Cytol. 2002;46(5):808-12.
Qureshi MN, Bolick D, Ringer PJ, Spagler FL, Zimmerman G. HPV testing in liquid cytology specimens: comparison of analytic sensitivity and specificity for in situ hybridization and chemiluminescent nucleic acid testing. Acta Cytol. 2005;49(2):120-6.
Qureshi MN, Rudelli RD, Tubbs RR, Biscotti CV, Layfield LJ. Role of HPV DNA testing in predicting cervical intraepithelial lesions: comparison of HC HPV and ISH HPV. Diagn Cytopathol. 2003;29(3):149-55.
Reid-Nicholson M, Gatscha RM, Riedel ER, Lin O. Atypical squamous cells, cannot exclude high grade intraepithelial lesion (ASC-H): does HPV matter? Diag Cytopathol. 2007;35(1):1-5.
Ronco G, Brezzi S, Carozzi F, Dalla Palma P, Giorgi-Rossi P, Minucci D, Naldoni C, Segnan N, Zappa M, Zorzi M, Cuzick J. The new technologies for cervical cancer screening randomized controlled trial. An overview of results during the first phase of recruitment. Gynecol Oncol. 2007;107(1) Supplement 1:S230-2.
Rowe LR, Aldeen W, Bentz JS. Prevalence and typing of HPV DNA by hybrid capture II in women with ASCUS, ASC-H, LSIL, and AGC on ThinPrep Pap tests. Diagn Cytopathol. 2004;30(6):426-32.
Sailors J, Gander R, Saboorian MH, Berkley P, Foster B, Ashfaq R. Stability of PreservCyt for Hybrid Capture (HC II) HPV test. Diagn Cytopathol. 2005;32(5):260-3.
Sarode VR, Werner C, Gander R, Foster B, Fulmer A, Saboorian MH, Ashfaq R. Reflex human papillomavirus DNA testing on residual liquid-based (TPPT) cervical samples. Cancer (Cancer Cytopathol). 2003;99(3):149-55.
Schnatz PF, Markelova NV, Holmes D, Mandavilli SR, O’Sullivan DM. The prevalence of cervical HPV and cytological abnormalities in association with reproductive factors of rural Nigerian women. J Women’s Health. 2008;17(2):279-85.
Sherman M, Castle P, Solomon D for the ASCUS LSIL Triage Group. Cervical cytology of atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion (ASC-H). Cancer Cytopathol. 2006;108(5):298-305.
Sherman ME, Schiffman M, Cox JT for the Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) group. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst. 2002;94(2):102-7.
Solomon D, Schiffman M, Tarone R, for the Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93(4):293-9.
Solomon D, Schiffman M, Tarone R, for the Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst. 2000;92:397-402.
Stoler MH, Schiffman M, for the Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500-5.
Susumu N, Aoki D, Noda T, Nagashima Y, Hirao T, Tamada Y, Banno K, Suzuki A, Suzuki N, Tsuda H, Inazawa J, Nozawa S. Diagnostic clinical application of two-color fluorescence in situ hybridization that detects chromosome 1 and 17 alterations to direct touch smear and liquid-based thin-layer cytologic preparations of endometrial cancers. Int J Gynecol Cancer. 2005;15(1):70-80.
Tarkowski TA, Rajeevan MS, Lee DR, Unger ER. Improved detection of viral RNA isolated from liquid-based cytology samples. Mol Diagn. 2001;6(2):125-30.
Wang-Johanning F, Lu DW, Wang Y, Johnson MR, Johanning G. Quantitation of human papillomavirus 16 E6 and E7 DNA and RNA in residual material from ThinPrep Papanicolaou Tests using real-time polymerase chain reaction analysis. Cancer. 2002; 94(8):2199-210.
Weaver EJ, Kovatich AJ, Bibbo M. Cyclin E expression and early cervical neoplasia in ThinPrep specimens. Acta Cytol. 2000;44:301-4.
Wright TC Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ for the ASCCP-sponsored Consensus Conference. 2001 consensus guidelines for the management of women with cervical cytologic abnormalities. JAMA. 2002;287(16):2120-9.
Wright TC Jr, Lorincz A, Ferris DG, Richart RM, Ferenczy A, Mielzynska I, Borgatta L. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol. 1998;178:962-6.
Xi LF, Toure P, Critchlow CW, Hawes SE, Dembele B, Sow PS, Kiviat NB. Prevalence of specific types of human papillomavirus and cervical squamous intraepithelial lesions in consecutive, previously unscreened, West-African women over 35 years of age. Int J Cancer. 2003;103(6):803-9.
Yang Y, Wang YF, Lang JH, Cheng XM, Li CJ, Shan Y, Yu M. [Clinical evaluation of high-risk HPV detection by hybrid capture II in screening cervical intraepithelial neoplasma]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28(3):395-8. [article in Chinese]
Yarkin F, Chauvin S, Konomi N, Wang W, Mo R, Bauchman G, Diaz A, Burstein D, Szporn A, Hauptman E, Zhang DY. Detection of HPV DNA in cervical specimens collected in cytologic solution by ligation-dependent PCR. Acta Cytol. 2003;47(3):450-6.
Yeoh G, Tse M, Chan K, Lord L. Human papillomavirus DNA and liquid-based cervical cytology cotesting in screening and follow-up patient groups. Acta Cytol. 2006;50(6):627-31.
Zhang W, Cohenford M, Lentrichia B, Isenberg HD, Simson E, Li H, Yi J, Zhang DY. Detection of chlamydia trachomatis by isothermal ramification amplification method: a feasibility study. J Clin Microbiol. 2002;40(1):128-32.
Zhao C, Austin M. Human papillomavirus DNA detection in ThinPrep Pap Test vials is independent of cytologic sampling of the transformation zone. Gynecol Oncol. 2007;107(2):231-5.
Zuna RE, Allen RA, Moore WE, Lu Y, Mattu R, Dunn ST. Distribution of HPV genotypes in 282 women with cervical lesions: evidence for three categories of intraepithelial lesions based on morphology and HPV type. Mod Pathol. 2006;Dec 22: [Epub ahead of print].
Zuna RE, Wang SS, Schiffman M, Solomon D. Comparison of human papillomavirus distribution in cytologic subgroups of low-grade squamous intraepithelial lesion. Cancer. 2006;108(5):288-97.
Zuna RE, Wang SS, Rosenthal DL, Jeronimo J, Schiffman M, Solomon D. Determinants of human papillomavirus-negative, low-grade squamous intraepithelial lesions in the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesions triage study (ALTS). Cancer (Cancer Cytopathol). 2005;105(5):253-62.
Zuna RE, Moore W, Dunn T. HPV DNA testing of the residual sample of liquid-based Pap test: utility as a quality assurance monitor. Mod Pathol. 2001;14(3):147-151.
Biscotti C, Dawson A, Dziura B, Galup L, Darragh T, Rahemtulla A, Wills-Frank L. Assisted primary screening using the automated ThinPrep Imaging System. Am J Clin Pathol. 2005;123(2):281-7.
Bolger N, Heffron C, Regan I, Sweeney M, Kinsella S, McKeown M, Creighton G, Russell J, O’Leary J. Implementation and evaluation of a new automated interactive image analysis system. Acta Cytol. 2006;50(5):483-91.
Chivukula M, Saad RS, Elishaev E, White S, Mauser N, Dabbs DJ. Introduction of the ThinPrep Imaging System (TIS): experience in a high volume academic practice. CytoJournal. 2007;4:6.
Cibas E, Hong X, Crum C, Feldman. Age-specific detection of high risk HPV DNA in cytologically normal computer-imaged ThinPrep Pap samples. Gynecol Oncol. 2007;104(3):702-6.
Dawson A. Clinical experience with the ThinPrep Imager System. Acta Cytol. 2006;50(5):481-2. Editorial.
Dawson AE. The changing face of cervical screening: challenges for the future. Diagn Cytopathol. 2005;33(2):63-4. Editorial.
Dawson A. Can we change the way we screen?: The ThinPrep Imaging System. Cancer (Cancer Cytopathol). 2004;102(6):340-4.
Davey E, d’Assuncao J, Irwig L, Macaskill P, Chan SF, Richards A, Farnsworth A. Accuracy of reading liquid based cytology slides using the ThinPrep Imager compared with conventional cytology: prospective study. British Medical Journal. 2007;335(7609):28.
Davey E, Irwig L, Macaskill P, Chan S, D’Annuncao J, Richards A, Farnsworth A. Cervical cytology reading times: a comparison between ThinPrep Imager and conventional methods. Diagn Cytol. 2007;35(9):550-4.
Dziura B, Quinn S, Richard K. Performance of an imaging system vs. manual screening in the detection of squamous intraepithelial lesions of the uterine cervix. Acta Ctyol. 2006;50(3):309-11.
Friedlander MA, Rudomina D, Lin O. Effectiveness of the ThinPrep Imaging System in the detection of adenocarcinoma of the gynecologic system. Cancer (Cancer Cytopathol). 2008;114(1):7-12.
Holund B, Grinsted P. Screening for cervical cancer in the country of Funen. Status of 25 years of development and experience. Ugeskr Laeger. 2006;168(22):2163-6. Article in Danish.
Lozano R. Comparison of computer-assisted and manual screening of cervical cytology. Gynecologic Oncology. 2007;104(3):702-6.
Miller FS, Nagel LE, Kenny-Moynihan MB. Implementation of the ThinPrep Imaging System in a high-volume metropolitian laboratory. Diagn Cytopathol. 2007;35(4):213-7.
Papillo JL, St. John TL, Leiman G. Effectiveness of the ThinPrep Imaging System: clinical experience in a low risk screening population. Diagn Cytopathol. 2008;38(3):155-60.
Roberts J, Thurloe J, Bowditch R, Hyne S, Greenberg M, Clarke J, Biro C. A three-armed trial of the ThinPrep Imaging System. Diagn Cytopathol. 2007;35(2):96-102.
Schledermann D, Hyldebrandt T, Ejersbo D, Hoelund B. Automated screening versus manual screening: a comparison of the ThinPrep Imaging System and manual screening in a time study. Diagn Cytopathol. 2007;35(6):348-52.
Zhang FF, Banks HW, Langford SM, Davey DD. Accuracy of ThinPrep Imaging System in detecting low-grade squamous intraepithelial lesions. Arch Pahtol Lab Med. 2007;131:773-6.
Zhao C, Elishaev E, Yuan K, Yu J, Austin RM. Very low human papillomavirus DNA prevalence in mature women with negative computer-imaged liquid-based Pap tests. Cancer (Cancer Cytopathol). 2007;111(5):292-7.
Bidus M, Maxwell GL, Kulasingam S, Rose GS, Elkas JC, Chernofsky M, Myers ER. Cost-effectiveness analysis of liquid-based cytology and human papillomavirus testing in cervical cancer screening. Obstet Gynecol. 2006;107(5):997-1005.
Braly P, Sedlacek T, Kinney W, Sheets EE, Walton L, Farber F, Cox JT. Reporting the potential benefits of new technologies for cervical cancer screening. J Low Gen Tract Dis. 2001;5(2):73-81.
Brown AD, et al. Cost-effectiveness of 3 methods to enhance the sensitivity of Papanicolaou testing. JAMA. 1999;28(4):347-53.
Cochand-Priollet B, Cartier I, de Cremoux P, Le Gales C, Ziol M, Molinie V, Petitjean A, Dosda A, Merea E, Biaggi A, Gouget I, Arkwright S, Vacher-Lavenu MC, Vielh P, Coste J. Cost-effectiveness of liquid-based cytology with or without hybrid-capture II HPV test compared with conventional Pap smears: a study by the French society of clinical cytology. Diagn Cytopathol. 2005;33(5):338-43.
Hutchinson ML et al. Clinical and cost implications of new technologies for cervical cancer screening: The impact of test sensitivity. Am J Manag Care. 2000;6:766-80.
Maxwell GL, Carlson JW, Ochoa M, Krivak T, Rose GS, Myers ER. Costs and effectiveness of alternative strategies for cervical cancer screening in military beneficiaries. Obstet Gynecol. 2002;100(4):740-8.
Merea E, Le Gales C, Cochand-Priollet B, Cartier I, de Cremoux P, Vacher-Levenu MC, Vielh P, Coste J. Cost of screening for cancerous and precancerous lesions of the cervix. Diagn Cytopathol. 2002;27(4):251-7.
Montz FJ, Farber FL, Bristow RE, Cornelison T. Impact of increasing Papanicolaou test sensitivity and compliance: A modeled cost and outcomes analysis. Obstet Gynecol. 2001;97:781-8.
Neville AM, Quinn MA. An alternative cost effectiveness analysis of ThinPrep in the Australian setting. Aust N Z J Obstet Gynaecol. 2005;45(4):289-94.
Sedlacek T, et al. Cervical cancer screening: what is cost effectiveness? J Low Gen Tract Dis. 1999;3:S1-S7.
Adams K, Absher K, Brill Y, Witzke D, Davey D. Reproducibility of subclassification of squamous intraepithelial lesions: conventional versus ThinPrep paps. J Low Gen Tract Dis. 2003;7(3):203-8.
Alves VA, Bibbo M, Schmitt FC, Milanezi F, Longatto Filho A. Comparison of manual and automated methods of liquid-based cytology. A morphologic study. Acta Cytol. 2004;48(2):187-93.
Baer A, Kiviat NB, Kulasingam S, Mao C, Kuypers J, Koutsky L. Liquid-based Papanicolaou smears without a transformation zone component: should clinicians worry? Obstet Gynecol. 2002;99(6):1053-9.
Banville N, Murray M, Turner L, Magee D, Gibbons D. The number of dyskaryotic cells on an initial ThinPrep cervical sample showing mild dyskaryosis has predictive value. Cytopathology. 2005;16(3):120-4.
Belsley NA, Tambouret RH, Musdraji J, Muzikansky A, Russell DK, Wilbur DC. Cytologic features of endocervical glandular lesions: comparison of SurePath, ThinPrep, and conventional smear specimen preparations. Diagn Cytopathol. 2008;36(4):232-7.
Bentz JS, Rowe LR, Gopez EV, Marshall CJ. The unsatisfactory ThinPrep Pap Test: missed opportunity for disease detection? Am J Clin Pathol. 2002;117:457-63.
Brogi E, Tambouret R, Bell DA. Classification of benign endometrial glandular cells in cervical smears from postmenopausal women. Cancer (Cancer Cytopathol). 2002;96(2):60-6.
Buccoliero AM, Castiglione F, Gheri CF, Garbibi F, Fambrini M, Bargelli G, Papparlardo S, Scarselli G, Marchionni M, Taddei GL. Liquid-based endometrial cytology: its possible value in postmenopausal asymptomatic women. Int J Gynecol Cancer. 2007;17:182-7.
Buccoliero AM, Gheri CF, Castiglione F, Garbini F, Barbetti A, Fambrini M, Bargelli G, Pappalardo S, Taddei A, Boddi V, Scarselli GF, Marchionni M, Taddei GL. Liquid-based endometrial cytology: cyto-histological correlation in a population of 917 women. Cytopathol. 2007;18:241-9.
Budge M, Halford J, Haran M, Mein J, Wright G. Comparison of a self-administered tampon ThinPrep test with conventional pap smears for cervical cytology. Aust N Z J Obstet Gynaecol. 2005;45(3):215-9.
Chacho MS, Mattie ME, Schwartz PE. Cytohistologic correlation rates between conventional Papanicolaou smears and ThinPrep cervical cytology: a comparison. Cancer (Cancer Cytopathol). 2003;99(3):135-40.
Chang BS, Pinkus GS, Cibas ES. Exfoliated endometrial cell clusters in cervical cytologic preparations are derived from endometrial stroma and glands. Am J Clin Pathol. 2006;125:77-81.
Dalton P, MacDonald S, Boerner S. Acetic acid recovery of gynecologic liquid-based samples of apparent low squamous cellularity. Acta Cytol. 2006;50(2):136-40.
Denton K. Liquid based cytology in cervical cancer screening is as sensitive as conventional cytology, and has other advantages. British Medical Journal. 2007; 335(7609):1-2.
Diaz-Rosario L, Kabawat S. Cell block preparation by inverted filter sedimentation is useful in the differential diagnosis of atypical glandular cells of undetermined significance in ThinPrep specimens. Cancer (Cancer Cytopathol). 2000;90(5):265-72.
Dowie R, Stoykova B, Crawford D, Desai M, Mather J, Morgan K, Shirt M. Liquid-based cytology can improve efficiency of cervical smear readers: evidence from timing surveys in two NHS cytology laboratories. Cytopathology. 2006;17(2):65-72.
Doyle B, O’Farrell C, Mahoney E, Turner L, Magee D, Gibbons D. Liquid-based cytology improves productivity in cervical cytology screening. Cytopathology. 2006;17(2):60-4.
Elsheikh TM, Kirkpatrick JL, Wu HH. The significance of “low-grade squamous intraepithelial lesion, cannot exclude high-grade squamous intraepithelial lesion” as a distinct squamous abnormality category in Papanicolaou tests. Cancer. 2006;108(5):277-81.
Eltabbakh GH et al. Significance of atypical glandular cells of undetermined significance of ThinPrep Papanicolaou smears. Gynecol Oncol. 2000;78(2):245-50.
Eltabbakh GH et al. Significance of atypical squamous cells of undetermined significance of ThinPrep Papanicolaou smears. Gynecol Oncol. 2000;79(1):44-9.
Feratovic R, Lewin S, Sonoda Y, Park KJ, Abu-Rustum NR, Moriera AL, Lin O. Cytologic findings after fertility-sparing radical trachelectomy. Cancer (Cancer Cytopathol). 2008;114(1):1-6.
Gu Y, Wu S, Meyer J, Hancock W, Burg L, Linder J, Hanlon D, Karger B. Proteomic analysis of high-grade dysplastic cervical cells obtained from ThinPrep slides using laser capture microdissection and mass spectometry. J Proteome Res. 2007;6(11):4256-68.
Haack LA, O’brien D, Selvaggi SM. Protocol for the processing of bloody cervical specimens: glacial acetic acid and the ThinPrep(R) Pap Test. Diagn Cytopathol. 2006;34(3):210-3 [Epub ahead of print]
Halford JA. Cytological features of chronic follicular cervicitis in liquid-based specimens: a diagnostic pitfall. Cytopathology. 2002;13:364-70.
Hecht JL, Sheets EE, Lee KR. Atypical glandular cells of undetermined significance in conventional cervical/vaginal smears and thin-layer preparations. Cancer (Cancer Cytopathol). 2002;96(1):1-4.
Henderson S, Stevens M, Walker T. Rapid review of liquid-based smears as a quality control measure. Diagn Cytopathol. 2004;31(3):141-6.
Hoerl HD, Roth-Cline MD, Shalkham JE, Pfister J, Stewart J 3rd, Guo Z, De Las Casas LE, Caya JG, Kurtycz DF. Rare atypical cells of undetermined significance (ASCUS): a clinically significant diagnosis? Diagn Cytopathol. 2002;27(1):5-9.
Hoerl HD et al. Screening parameters for ThinPrep and conventional gynecologic cytology via automated monitoring. Acta Cytol. 2000;44:618-624.
Holton T, Smith D, Terry M, Madgwick A, Levine T. The effect of lubricant contamination on ThinPrep cervical cytology liquid-based preparations. Cytopathol. 2007; December 18 [Epub ahead of print].
Holund B, Grinsted P. [Screening for cervical cancer in the county of Funen. Status of 25 years of development and experiences]. Ugeskr Laeger. 2006;168(22):2163-6. [article in Danish]
Kabbani W, Raisanen J, Thomas S, Saboorian M, Ashfaq R. Cell block findings from residual Preservcyt samples in unsatisfactory ThinPrep paps: no additional benefit. Diagn Cytopathol. 2002;27(4):238-43.
Kapanis K, Kiziridou A, Liberis V, Destouni C, Galazios. E-cadherin expression during progression of squamous intraepithelial lesions of the uterine cervix. Eur J Gynaecol Oncol. 2005;26(6):608-10.
Keyhani-Rofagha S, Vesey-Shecket M. Diagnostic value, feasibility, and validity of preparing cell blocks from fluid-based gynecologic cytology specimens. Cancer (Cancer Cytopathol). 2002;96(4):204-9.
Kothari A, Karim SZ, Gordon A, Raslan F, Abdalla M, George S. A comparative study of two devices used for cervical cell sampling raises some doubts about liquid-based cytology. Int J Gynecol Cancer. 2006;16:1579-86.
Kumar N, Massimo Bongiovanni M, Molliet MJ, Pelte MF, Egger JF, Pache JC. Reclassification and analysis of clinifal significance of atypical glandular cells on ThinPrep using The Bethesday 2001: Geneva experience. Swiss Med Wkly. 2007;137:635-41.
Kyroudi A, Papaefthimiou M, Symiakaki H, Mentzelopoulou P, Voulgaris Z, Karakitsos P. Increasing diagnostic accuracy with a cell block preparation from ThinLayer endometrial cytology. Acta Cytol. 2006;50(1):63-9.
Maguire A, Turner L, Magee D, Gibbons D. Decrease in numbers of glandular cell groups in post-LLETZ liquid-based cytology preparations. Cytopathol. 2008;19:44-7.
Maksem JA, Dhanwada V, Trueblood JE, Weidmann J, Kane B, Bolick DR, Bedrossian CW, Kurtycz DF, Stewart J 3rd. Testing automated liquid-based cytology samples with a manual liquid-based cytology method using residual cell suspensions from 500 ThinPrep cases. Diag Cytopathpol. 2006;34(6):391-6.
McQueen F, Duvall E. Using a quality control approach to define an ‘adequately cellular’ liquid-based cervical cytology specimen. Cytopathology. 2006;17(4):168-74.
Morrison C, Prokorym P, Piquero C, Wakely PE, Nuovo GJ. Oral contraceptive pills are associated with artifacts in ThinPrep Pap smears that mimic low-grade squamous intraepithelial lesions. Cancer (Cancer Cytopathol). 2003;99(2):75-82.
Negri G, Menia E, Egarter-Vigl E, Vittadello F, Mian C. ThinPrep versus conventional Papanicolaou smear in the cytologic follow-up of women with equivocal cervical smears. Cancer (Cancer Cytopathol). 2003;99(6):342-5.
Ozkan F, Ramzy I, Mody DR. Glandular lesions of the cervix on thin-layer Pap tests. Validity of cytologic criteria used in identifying significant lesions. Acta Cytol. 2004;48(3):372-9.
Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, Shepherd BE, Hicks ML, Stringer EM, Vermund SH. Prevalence and predictors of squamous intraepithelial lesions of the cervix in HIV-infected women in Lusaka, Zambia. Gynecol Oncol. 2006;103(3):1017-22.
Polina R, Sturgis C, Patterson J, Patterson BK. Rapid, high throughput determination of cervical cytology specimen adequacy using a capillary-based cytometer. Cytometry Part B: Clinical Cytometry 2007; November 28 [Epub ahead of print].
Quddus MR, Sung CJ, Eklund MJ, Zhang C, Steinhoff MM. Atypical squamous cells, cannot exclude HSIL: a review of original vs. duplicate thin-layer slides. J Reprod Med. 2004;49(6):457-62.
Quddus MR, Sung CJ, Steinhoff MM, Lauchlan SC, Singer DB, Hutchinson ML. Atypical squamous metaplastic cells: reproducibility, outcome, and diagnostic features on ThinPrep Pap Test. Cancer (Cancer Cytopathol). 2001;93:16-22.
Raab SS, Grzybicki DM, Hart AR, Kiely S, Andrew-Jaja C, Scioscia E. Willingness to pay for new Papanicolaou test technologies. Am J Clin Pathol. 2002;117:524-33.
Ramsaroop R, Chu I. Accuracy of diagnosis of atypical glandular cells-conventional and ThinPrep. Diagn Cytopathol. 2006;34(9):614-9.
Renshaw AA, Mody DR, Walsh M, Bentz JS, Colgan TJ; Cytopathology Resource Committee, College of American Pathologists. The significance of certification in liquid-based cytology and performance in the college of american pathologists interlaboratory comparison program in cervicovaginal cytopathology. Arch Pathol Lab Med. 2006;130(9):1269-72.
Renshaw AA, Mody DR, Styer P, Schwartz M, Ducatman B, Colgan TJ. Papanicolaou tests with mixed high-grade and low-grade squamous intraepithelial lesion features: distinct performance in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytopathology. Arch Pathol Lab Med. 2006;130(4):456-9.
Renshaw AA, Mody DR, Wang E, Haja J, Colgan T. Hyperchromatic crowded groups in cervical cytology – differing appearances and interpretations in conventional and ThinPrep preparations. Arch Pathol Lab Med. 2006;130:332-6.
Richards K, Dziura B, Hornish A. Cell block preparation as a diagnostic technique complementary to fluid-based monolayer cervicovaginal specimens. Acta Cytol. 1999;43(1):69-73.
Rowe LR, Bentz RS. A simple method to determine the need for glacial acetic acid treatment of bloody ThinPrep Pap test before slide processing. Diagn Cytopathol. 2004;31(5):321-5.
Selvaggi SM. Background features of endometrial carcinoma on ThinPrep cytology. Diagn Cytopathol. 2005;33(3):162-5.
Sherman ME, Kahler J, Gustafson KS, Wang SS. “Sip volume” as a quality indicator in liquid-based cervical cytology. Cancer (Cancer Cytopathol). 2006;108(6):462-7.
Slater DN, Rice S, Stewart R, Melling SE, Hewer EM, Smith JH. Proposed Sheffield quantitative criteria in cervical cytology to assist the grading of squamous cell dyskaryosis, as the British Society for Clinical Cytology definitions require amendment. Cytopathology. 2005;16(4):179-92.
Snyder T, Renshaw A, Styler P, Mody D, Colgan T. Altered recognition of reparative changes in ThinPrep specimens in the College of American Pathologists Gynecologic Cytology programs. Arch Pathol Lab Med. 2005;129(7):861-5.
Sweeney BJ, Haq Z, Happel JF, Weinstein B, Schneider D. Comparison of the effectiveness of two liquid-based Papanicolaou systems in the handling of adverse limiting factors, such as excessive blood. Cancer (Cancer Cytopathol). 2006;108(1):27-31.
Young NA, Moriarty AT, Walsh MK, Wang E, Wilbur DC, College of American Pathologists. The potential for failure in gynecologic regulatory proficiency testing with current slide validation criteria: results from the College of American Pathologists Interlaboratory Comparison in Gynecologic Cytology Program. Arch Pathol Lab Med. 2006;130(8):1114-8.
Zhou J, Tomashefski JF, Khiyami A. ThinPrep Pap Tests in patients with endometrial cancer: a histo-cytological correlation. Diagn Cytopathol. 2007;35(7):448-53.
Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cytology. Obstet Gynecol. 2008;111:167-77.
Belinson J, Qiao YL, Pretorius R, Zhang WH, Elson P, Li L, Pan QJ, Fischer C, Lorincz A, Zahniser D. Shanxi province cervical cancer screening study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol. 2001;83(2):439-44.
Bolger N, Heffron C, Regan I, Sweeney M, Kinsella S, McKeown M, Creighton G, Russell J, O’Leary J. Implementation and evaluation of a new automated interactive image analysis system. Acta Cytol. 2006;50(5):483-91.
Cheung AN, Szeto EF, Leung BS, Khoo US, Ng AW. Liquid-based cytology and conventional cervical smears: a comparison study in an Asian screening population. Cancer. 2003 Dec 25;99(6):331-5.
Clavel, Masure M, Bory JP, Putaud I, Mangeonjean C, Lorenzato M, Nazeyrollas P, Gabriel R, Quereux C, Birembault P. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7,932 women. Br J Cancer. 2001; 89(12):1616-23.
Cremer M, de LasCasas L, Kurtycz DFI, Schink J. Liquid-based cytology specimen studies to screen for cervical dysplasia in rural El Salvador. Int J Gynecol & Obstet. 2005 June 16; Epub ahead of print.
Cubie HA, Moore C, Waller M, Moss S; National Cervical Screening Committee LBC/HPV Pilot Steering Group. The development of a quality assurance programme for HPV testing within the UK NHS cervical screening LBC/HPV studies. J Clin Virol. 2005;33(4):287-92.
Davey E, d’Assuncao J, Irwig L, Macaskill P, Chan SF, Richards A, Farnsworth A. Accuracy of reading liquid based cytology slides using the ThinPrep Imager compared with conventional cytology: prospective study. British Medical Journal. 2007;335(7609):28.
Ferreccio C, Bratti MC, Sherman ME, et al. A comparison of single and combined visual, cytologic, and virologic tests as screening strategies in a region at high risk of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:815-23.
Grace A, McBrearty P, Troost S, Thornhill M, Kay E, Leader M. Comparative study: conventional cervical and ThinPrep Pap tests in a routine clinical setting. Cytopathology. 2002;13(4):200-5.
Hoelund B. Implementation of liquid-based cytology in the screening programme against cervical cancer in the County of Funen, Denmark, and status for the first year. Cytopathology. 2003; 14(5):269-74.
Holund B, Grinsted P. [Screening for cervical cancer in the county of Funen. Status of 25 years of development and experiences]. Ugeskr Laeger. 2006;168(22):2163-6. Danish.
Hutchinson ML, et al. Utility of liquid-based cytology for cervical carcinoma screening: results of a population-based study conducted in a region of Costa Rica with a high incidence of cervical carcinoma. Cancer (Cancer Cytopathol). 1999; 87(2):48-55.
Klinkhamer PJ, Meerding WJ, Rosier PF, Hanselaar AG. Liquid-based cervical cytology. Cancer. 2003;99(5):263-71.
Kothari A, Karim SZ, Gordon A, Raslan F, Abdalla M, George S. A comparative study of two devices used for cervical cell sampling raises some doubts about liquid-based cytology. Int J Gynecol Cancer. 2006;16:1579-86.
Kumar N, Massimo Bongiovanni M, Molliet MJ, Pelte MF, Egger JF, Pache JC. Reclassification and analysis of clinifal significance of atypical glandular cells on ThinPrep using The Bethesday 2001: Geneva experience. Swiss Med Wkly. 2007;137:635-41.
Liverani M, De Paola F, Danesi S, Fedriga R, Dalbuoni V, Agnoletti R, Amadori AR, Mirra F, Bonoli W, Saragoni L. [Applicative aspects of liquid-based cytology in cervical cancer screening]. Pathologica. 2006;98(6):629-34. [article in Italian]
Luthra, Chishti M, Dey P, Jolly SV, Abdulla M, Das DK, Sugathan TN, Ajrawi MT, George J, George SS, Aziz AA, Al-Juwaiser A, Karim FA, Mallik MK, Sheikh ZA, Khan S. Performance of monolayered cervical smears in a gynecology outpatient setting in Kuwait. Acta Cytol. 2002;46(2):303-10.
Malle D, Pateinakis P, Chakka E, Destouni C. Experience with a thin-layer, liquid-based cervical cytologic screening method. Acta Cytol. 2003;47(2):129-34.
Merea E, Le Gales C, Cochand-Priollet B, Cartier I, de Cremoux P, Vacher-Levenu MC, Vielh P, Coste J. Cost of screening for cancerous and precancerous lesions of the cervix. Diagn Cytopathol. 2002;27(4):251-7.
Monsonego J, et al. Liquid-based cytology for primary cervical cancer screening: a multi-centre study. Br J Cancer. 2001;84(3):360-6.
Murphy N, Ring M, Killalea AG, Uhlmann V, O’Donovan M, Mulcahy F, Turner M, McGuinness E, Griffin M, Martin C, Sheils O, O’Leary JJ. p16INK4A as a marker for cervical dyskaryosis: CIN and cGIN in cervical biopsies and ThinPrep smears. J Clin Pathol. 2003;56(1):56-63.
Nam JH, Kim HS, Lee JS, Choi HS, Min KW, Park CS. A comparison of modified MonoPrep2 of liquid-based cytology with ThinPrep Pap test. Gynecol Oncol. 2004 Sep;94(3):693-8.
Obwegeser JH, Brack S. Does liquid-based technology really improve detection of cervical neoplasia? A prospective, randomized trial comparing the ThinPrep Pap Test with the conventional Pap Test, including follow-up of HSIL cases. Acta Cytol. 200;45(5):709-14.
Pan Q, Belinson JL, Li L, Pretorius RG, Qiao YL, Zhang WH, Zhang X, Wu LY, Rong SD, Sun YT. A thin-layer, liquid-based Pap test for mass screening in an area of China with a high incidence of cervical carcinoma: a cross-sectional, comparative study. Acta Cytol. 2003;47(1):45-50.
Pan Q, Li L, Qiao Y. Comparative study on liquid-based cytology for cervical carcinoma screening in high-risk area of China. Zhonghua Zhong Liu Za Zhi. 2001 Jul;23(4):309-12.
Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, Shepherd BE, Hicks ML, Stringer EM, Vermund SH. Prevalence and predictors of squamous intraepithelial lesions of the cervix in HIV-infected women in Lusaka, Zambia. Gynecol Oncol. 2006 Jul 26; [Epub ahead of print]
Park IA, Lee SN, Chae SW, Park KH, Kim JW, Lee HP. Comparing the accuracy of ThinPrep Pap tests and conventional Papanicolaou smears on the basis of the histologic diagnosis: a clinical study of women with cervical abnormalities. Acta Cytol. 2001;45(4):525-31.
Qian DY, Cen JM, Wang D, Zeng RH, Lin AH, Shu YH, Hong DH, Huang ZH. Combining high-risk human papillomavirus DNA test and cytological test to detect early cervical dysplasia. Zhonghua Fu Chan Ke Za Zhi. 2006;41(1):34-7. [article in Chinese]
Rahimpanah F, Reid RI. Fallopian tube carcinoma detected by ThinPrep cytology smear. Med J Aust. 2000;17(1):38.
Ring M, Bolger N, O’Donnell M, Malkin A, Bermingham N, Akpan E, Mulcahy F, Turner NJ, Griffin M, O’Leary JJ. Evaluation of liquid-based cytology in cervical screening of high-risk populations: a split study of colposcopy and genito-urinary medicine populations. Cytopathology. 2002;13:152-9.
Roberts JM, Gurley AM, Thurloe JK, Bowditch R, Laverty CR. Evaluation of the ThinPrep Pap Test as an adjunct to the conventional Pap smear. Med J Aust. 1997;167:466-7.
Roberts JM, Thurloe JK, Bowditch RC, Humcevic J, Laverty CR. Comparison of ThinPrep and Pap smear in relation to prediction of adenocarcinoma in situ. Acta Cytol. 1999;43(1):74-80.
Schledermann D, Ejersbo D, Hoelund B. Improvement of diagnostic accuracy and screening conditions with liquid-based cytology. Diagn Cytopathol. 2006;34(11):780-5.
Schledermann D, Ejersbo D, Hoelund B. Significance of atypia in conventional Papanicolaou smears and liquid-based cytology: a follow-up study. Cytopathology. 2004 Jun;15(3):148-53.
Schledermann D, Hyldebrandt T, Ejersbo D, Hoelund B. Automated screening versus manual screening. a comparison of the ThinPrep Imaging System and manual screening in a time study. Diagn Cytopathol. 2007;35(6):348-52.
Schnatz PF, Markelova NV, Holmes D, Mandavilli SR, O’Sullivan DM. The prevalence of cervical HPV and cytological abnormalities in association with reproductive factors of rural Nigerian women. J Women’s Health. 2008;17(2):279-85.
Shield PW et al. Improving cervical cytology screening in a remote, high risk population. Med J Aust. 1999;170(6):255-8.
Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, Wright TC Jr. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2005;118(4):957-62.
Tuncer ZS, Basaran M, Sezgin Y, Firat P, Mocan Kuzey G. Clinical results of a split sample liquid-based cytology (ThinPrep) study of 4,322 patients in a Turkish institution. Eur J Gynaecol Oncol. 2005;26(6):646-8.
Wang TY et al. Comparison of fluid-based, thin-layer processing and conventional Papanicolaou methods for uterine cervical cytology. J Formos Med Assoc. 1999;98(7):500-5.
Weintraub J et al. Efficacy of a liquid-based thin layer method for cervical cancer screening in a population with a low incidence of cervical cancer. Diagn Cytopathol. 2000;22(1):52-9.
Yang Y, Wang YF, Lang JH, Cheng XM, Li CJ, Shan Y, Yu M. [Clinical evaluation of high-risk HPV detection by hybrid capture II in screening cervical intraepithelial neoplasma]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006 Jun;28(3):395-8. [article in Chinese] Yeoh GPS et al. Evaluation of the ThinPrep Papanicolaou test in clinical practice: six-month study of 16, 541 cases with histological correlation in 220 cases. HKMJ. 1999;5:233-9.
Yeoh GPS et al. Evaluation of the ThinPrep Papanicolaou test in clinical practice: six-month study of 16, 541 cases with histological correlation in 220 cases. HKMJ. 1999;5:233-9.
Related Products

Overview
Package Inserts
Leading the charge in cervical disease prevention with the ThinPrep system and Aptima HPV assays
Hologic has remained an unwavering advocate for women’s health for more than two decades. Our goals as a company are intrinsically tied to changes in best-practices for women’s health, applying the latest findings in diagnostic medicine to the development of new products and technologies in response to the emergence of new discoveries in medicine.
Cervical disease screening is an essential component of our efforts in women’s health. Hologic is the leader in Pap and human papillomavirus (HPV) testing. The ThinPrep Pap test helps healthcare providers detect the presence of abnormal cervical cells, and the Aptima HPV assays identify high-risk HPV mRNA that is indicative of the HPV infections most likely to lead to cervical disease.1-3
Today, screening with Pap+HPV Together (co-testing) provides the best possible protection against cervical cancer for women ages 30-65.4-6 Now, what was once a top cancer among women is up to 93% preventable.7
The introduction of the Pap smear and, later the ThinPrep Pap test, have contributed to a decline in cervical cancer rates of more than 60% since the 1950s.1,8 Since then, HPV has been identified as a cause of cervical cancer, and HPV testing and vaccinations have been developed.9
These medical triumphs have fortified the medical community’s ability to detect and prevent cervical disease and cancer. Today, the combination of data-supported guidelines for cervical cancer screening and the availability of HPV vaccination are key in the fight for women’s health.
Hologic released the first liquid-based cytology option in cervical disease screening in 1996: the ThinPrep Pap test.1 Today, more than 20 years after the release of the ThinPrep Pap test, it remains the preferred choice in Pap testing in the United States.10 ThinPrep Pap tests account for more than 80% of Pap tests performed in the United States, with 650 million tests performed globally so far.10
More than 170 scientific studies involving the ThinPrep Pap test have demonstrated its benefits, including increased disease detection, reduction of equivocal diagnoses, improved specimen adequacy, adjunctive molecular testing and morphology assessment.11
The College of American Pathologists reported increased HSIL (high-grade squamous intraepithelial lesions) and LSIL (low-grade squamous intraepithelial lesions) in labs using LBC versus labs that utilized conventional Pap testing.12 It also showed increased sensitivity for cervical adenocarcinoma over conventional Pap testing.13
The Aptima HPV assay and Aptima HPV 16 18/45 genotype assay target HPV types that pose the largest threat to women.2,3 While other HPV assays target DNA, the Aptima HPV assays target mRNA, which studies show reflects the presence and activity of high-risk HPV infection.2,3 The Aptima HPV assay detects E6/E7 mRNA, which is indicative of those HPV infections more likely to cause cervical disease.2 The Aptima HPV 16 18/45 genotype assay identifies HPV types 16, 18 and 45, which are associated with up to 80% of all invasive cervical cancers worldwide.3,14-15
Screening women with Aptima HPV assays helps providers optimize care for each patient.
The ThinPrep Pap test is just one of several offerings in the ThinPrep system. The ThinPrep processors, imagers and review scopes help improve workflow in laboratories and assist with cytotechnologists’ ability to identify abnormalities. These instruments help complete the ThinPrep system and maximize laboratory efficiency and accuracy in disease detection.
In addition to the ThinPrep Pap test, Hologic remains committed to providing laboratories with innovative and effective cytology solutions for non-gynecological testing needs.
Processors
Imagers and Review Scopes
Combines the power of ThinPrep imaging and manual microscope review in a single desktop system, with slides imaged and ready for review in approximately 90 seconds.
Identifies 22 areas of interest within the ThinPrep Pap test slides with the largest, darkest nuclei for cytotechnologists to examine.
Same trusted algorithm as the ThinPrep image processor, but with a faster camera, expanded label-reading formats and on-demand imaging.
Presents the 22 fields of view to the cytotechnologist for their review.
Combines the power of the review scope with the flexibility of a manual microscope.
Stainers
Now Available Compass Stainer: Adds flexibility to both routine and special staining procedures. Comes programmed with the ThinPrep Stain Protocol for customer convenience.
From Pap and HPV testing to our full range of diagnostic products and cross-divisional innovations like the Genius™ 3D Mammography exam for breast health, Hologic delivers for women. Our promise to offer innovative products to meet the needs of women globally persists throughout all that we do, including in cervical disease screening. Working together, we can achieve the goal of defeating cervical disease.